Masticatory Motor Pattern in the Koala (Phascolarctos Cinereus)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
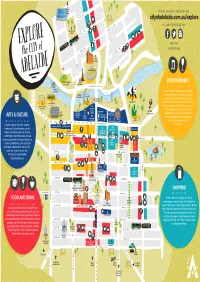
Cityofadelaide.Com.Au/Explore RYMILL NATIONAL NATIONAL
TO LE FEVRE TCE ADELAIDE AQUATIC FOR TIPS ON WHAT TO SEE AND DO VISIT CENTRE TYNTE ST cityofadelaide.com.au/explore FOLLOW CITYOFADELAIDE ON: WELLINGTON KINGSTON TCE SQUARE ARCHER ST O’CONNELL ST STANLEY ST #ADELAIDE #VISITADELAIDE WARD ST MELBOURNE ST ST PETER’S CATHEDRAL JEFFCOTT ST ADELAIDE OVAL NORTH ADELAIDE COLONEL ENTERTAINMENT GOLF COURSE LIGHT STATUE WAR MEMORIAL DRIVE TORRENS RIVER WEIR Wander through the yellow areas of the map TORRENS for a range of things to see and do for all ages, FOOTBRIDGE ROTUNDA including music, comedy, theatre, sport and POPEYE ELDER PARK ADELAIDE ZOO recreation. Check out a game at the Adelaide Oval, OLD ADELAIDE LAUNCH GAOL BONYTHON PARK take a cruise along the River Torrens, play a round PLAYSPACE ADELAIDE CASINO FESTIVAL at the North Adelaide Golf Course, visit the GOVERNMENT NATIONAL CENTRE ADELAIDE HOUSE MIGRATION WINE CENTRE Adelaide Zoo or catch a live band. There is SAHMRI CONVENTION NATIONAL MUSEUM RAILWAY ARTS & CULTURE CENTRE WAR MEMORIAL always something entertaining happening STATION BOTANIC PARLIAMENT AVE KINTORE ART GARDENS HOUSE LIBRARY MUSEUM GALLERY in the City, day and night and all NORTH TCE year round! A diverse range of museums, galleries, SAMSTAG MUSEUM LION ARTS CENTRE theatres and cultural landmarks can be MALLS BALLS AYERS HOUSE MALLS PIGS JAM FACTORY ST FROME found in the dark blue areas of the map. MERCURY CINEMA BANK ST GAWLER PL GAWLER ST PULTENEY HINDLEY ST MEDIA RESOURCE CENTRE A stroll through any of these areas will also RUNDLE MALL uncover quirky street art, famous statues and RUNDLE ST sculptures. -

Red-Necked Wallaby (Bennett’S Wallaby) Macropus Rufogriseus
Red-necked Wallaby (Bennett’s Wallaby) Macropus rufogriseus Class: Mammalia Order: Diprotodontia Family: Macropodidae Characteristics: Red-necked wallabies get their name from the red fur on the back of their neck. They are also differentiated from other wallabies by the white cheek patches and larger size compared to other wallaby species (Bioweb). The red-necked wallaby’s body fur is grey to reddish in color with a white or pale grey belly. Their muzzle, paws and toes are black (Australia Zoo). Wallabies look like smaller kangaroos with their large hindquarters, short forelimbs, and long, muscular tails. The average size of this species is 27-32 inches in the body with a tail length of 20-28 inches. The females weigh about 25 pounds while the males weigh significantly more at 40 pounds. The females differ from the males of the species in that they have a forward opening pouch (Sacramento Zoo). Range & Habitat: Flat, high-ground eucalyptus Behavior: Red-necked wallabies are most active at dawn and dusk to avoid forests near open grassy areas in the mid-day heat. In the heat, they will lick their hands and forearms to Tasmania and South-eastern promote heat loss. (Animal Diversity) These wallabies are generally solitary Australia. but do forage in small groups. The males will have boxing matches with one another to determine social hierarchy within populations. They can often be seen punching, wrestling, skipping, dancing, standing upright, grabbing, sparring, pawing, and kicking. All members of the kangaroo and wallaby family travel by hopping. Red-necked wallabies can hop up to 6 feet in the air. -

Tammar Wallaby Macropus Eugenii (Desmarest, 1817)
Tammar Wallaby Macropus eugenii (Desmarest, 1817) Description Dark, grizzled grey-brown above, becoming rufous on the sides of the body and the limbs, especially in males. Pale grey-buff below. Other Common Names Dama Wallaby (South Australia) Distribution The Western Australian subspecies of the Tammar Wallaby was previously distributed throughout most of the south-west of Western Australia from Kalbarri National Park to Cape Arid on the south coast Photo: Babs & Bert Wells/DEC and extending to western parts of the Wheat belt. Size The Tammar Wallaby is currently known to inhabit three islands in the Houtman Abrolhos group (East and West Wallabi Island, and an introduced population on North Island), Garden Island near Perth, Kangaroo Island wallabies Middle and North Twin Peak Islands in the Archipelago of the Head and body length Recherche, and several sites on the mainland - including, Dryandra, Boyagin, Tutanning, Batalling (reintroduced), Perup, private property 590-680 mm in males near Pingelly, Jaloran Road timber reserve near Wagin, Hopetoun, 520-630 mm in females Stirling Range National Park, and Fitzgerald River National Park. The Tammar Wallaby remains relatively abundant at these sites which Tail length are subject to fox control. 380-450 mm in males They have been reintroduced to the Darling scarp near Dwellingup, 330-440 mm in females Julimar Forest near Bindoon, state forest east of Manjimup, Avon Valley National Park, Walyunga National Park, Nambung National Park and to Karakamia and Paruna Sanctuaries. Weight For further information regarding the distribution of this species Western Australian wallabies please refer to www.naturemap.dec.wa.gov.au 2.9-6.1 kg in males Habitat 2.3-4.3 kg in females Dense, low vegetation for daytime shelter and open grassy areas for feeding. -

Ecology of the Koala, Phascolarctos Cinereus
I give eonsent to this eopy of ny thesis, r,,rhen d.eposited. in the Universit.y Library, being avail-abl-e 1'or loan and. photocopying. Date . ?! ÛP,"+ .13:r.o.. S igned. CONTENTS SUM MA RY ACKNOWLEDGEMENTS lil INTRODUCTION I PA,RT I FIELD STUDIES INTRODUCTION O.l Kongoroo lslqnd B O.2 Floro ond Founo il 0.3 Philpott's Study l3 O.4 Methods t5 0.5 Results 25 I THE DISTRIBUTION AND ABUN DANCE OF KOALAS I. I The Distribution of Koalos 29 | .2 The Abundonce of Koo lqs 34 2 BREEDING, GROWTH AND DEVELOPA,\E¡.¡T 2.1 Breeding 39 2.2 Pouch Young 40 2.3 Growth, Ageing ond LongevitY 49 2.4 Sexucrl Moturity 54 I SUMMARY The distribution of koalas u'ithin Flinders Chase was fou-nd to be made up of areas centred on the occurrences of manna guilr , Euca.ly¡rtus viminalis. Some koalas br:owsed chiefly iri trees of other species but tlrere liÌere ferv animals, if any, that clid not feed on the foliage of E. r'iminalis rnore or less regularly. The composition of populations in sever¿rl sürcly areas changed from üirne to time but over aE long as three successir¡e years of observat:lorr the numhers remained ::emarkably constant. The koalas bred in the surnmer: arrd early auturnn, and a high proporüon of feinales successfully raised a single young to independence each year. Growth of the yourìg was :lapid over the first Lhree yearr!; it slowed. down thereafter and anirnals reached firll size in tlieir fourth and fiffh years. -

Encouraging Possums
Encouraging Possums Keywords: possums, mammals, habitat, management, nest boxes Location: southwest Author: Emma Bramwell Possums are delightful and appealing creatures, with THE SEVEN SPECIES their soft downy fur and large innocent eyes. Some may be as small as a mouse while others are the size of a domestic • Honeypossum Tarsipes rostratus cat. The honey possum is the smallest of the Western The western ringtail and common brushtail possums are Australian possums, and is endemic to (occurring only in) two of the most commonly seen native animals around urban the lower southwest, in heaths with a rich diversity of areas in the southwest of Western Australia. The common nectar-producing plants. brushtail possum in particular has adapted to urban Mainly nocturnal, the honey possum sleeps during the development, and readily takes up residence in human day in hollow stems or abandoned bird nests, emerging at dwellings. With careful planning and management, people night to feed on the nectar and pollen that exclusively make can live harmoniously with these creatures and enjoy the up its diet, probing flowers with its long, pointed snout and close proximity of wildlife. brush-tipped tongue. In colder weather the honey possum becomes torpid (semi-hibernates). The honey possum has no obvious breeding season. WHAT IS A POSSUM? Most young are produced when pollen and nectar are most abundant, and females usually raise two or three young at a A number of small to medium-sized, tree-climbing time. Australian marsupial species have been given the common Provided large areas of habitat are retained, the honey name of possum. -

Macropod Herpesviruses Dec 2013
Herpesviruses and macropods Fact sheet Introductory statement Despite the widespread distribution of herpesviruses across a large range of macropod species there is a lack of detailed knowledge about these viruses and the effects they have on their hosts. While they have been associated with significant mortality events infections are usually benign, producing no or minimal clinical effects in their adapted hosts. With increasing emphasis being placed on captive breeding, reintroduction and translocation programs there is a greater likelihood that these viruses will be introduced into naïve macropod populations. The effects and implications of this type of viral movement are unclear. Aetiology Herpesviruses are enveloped DNA viruses that range in size from 120 to 250nm. The family Herpesviridae is divided into three subfamilies. Alphaherpesviruses have a moderately wide host range, rapid growth, lyse infected cells and have the capacity to establish latent infections primarily, but not exclusively, in nerve ganglia. Betaherpesviruses have a more restricted host range, a long replicative cycle, the capacity to cause infected cells to enlarge and the ability to form latent infections in secretory glands, lymphoreticular tissue, kidneys and other tissues. Gammaherpesviruses have a narrow host range, replicate in lymphoid cells, may induce neoplasia in infected cells and form latent infections in lymphoid tissue (Lachlan and Dubovi 2011, Roizman and Pellet 2001). There have been five herpesvirus species isolated from macropods, three alphaherpesviruses termed Macropodid Herpesvirus 1 (MaHV1), Macropodid Herpesvirus 2 (MaHV2), and Macropodid Herpesvirus 4 (MaHV4) and two gammaherpesviruses including Macropodid Herpesvirus 3 (MaHV3), and a currently unclassified novel gammaherpesvirus detected in swamp wallabies (Wallabia bicolor) (Callinan and Kefford 1981, Finnie et al. -

Platypus Collins, L.R
AUSTRALIAN MAMMALS BIOLOGY AND CAPTIVE MANAGEMENT Stephen Jackson © CSIRO 2003 All rights reserved. Except under the conditions described in the Australian Copyright Act 1968 and subsequent amendments, no part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, mechanical, photocopying, recording, duplicating or otherwise, without the prior permission of the copyright owner. Contact CSIRO PUBLISHING for all permission requests. National Library of Australia Cataloguing-in-Publication entry Jackson, Stephen M. Australian mammals: Biology and captive management Bibliography. ISBN 0 643 06635 7. 1. Mammals – Australia. 2. Captive mammals. I. Title. 599.0994 Available from CSIRO PUBLISHING 150 Oxford Street (PO Box 1139) Collingwood VIC 3066 Australia Telephone: +61 3 9662 7666 Local call: 1300 788 000 (Australia only) Fax: +61 3 9662 7555 Email: [email protected] Web site: www.publish.csiro.au Cover photos courtesy Stephen Jackson, Esther Beaton and Nick Alexander Set in Minion and Optima Cover and text design by James Kelly Typeset by Desktop Concepts Pty Ltd Printed in Australia by Ligare REFERENCES reserved. Chapter 1 – Platypus Collins, L.R. (1973) Monotremes and Marsupials: A Reference for Zoological Institutions. Smithsonian Institution Press, rights Austin, M.A. (1997) A Practical Guide to the Successful Washington. All Handrearing of Tasmanian Marsupials. Regal Publications, Collins, G.H., Whittington, R.J. & Canfield, P.J. (1986) Melbourne. Theileria ornithorhynchi Mackerras, 1959 in the platypus, 2003. Beaven, M. (1997) Hand rearing of a juvenile platypus. Ornithorhynchus anatinus (Shaw). Journal of Wildlife Proceedings of the ASZK/ARAZPA Conference. 16–20 March. -

A Dated Phylogeny of Marsupials Using a Molecular Supermatrix and Multiple Fossil Constraints
Journal of Mammalogy, 89(1):175–189, 2008 A DATED PHYLOGENY OF MARSUPIALS USING A MOLECULAR SUPERMATRIX AND MULTIPLE FOSSIL CONSTRAINTS ROBIN M. D. BECK* School of Biological, Earth and Environmental Sciences, University of New South Wales, Sydney, New South Wales 2052, Australia Downloaded from https://academic.oup.com/jmammal/article/89/1/175/1020874 by guest on 25 September 2021 Phylogenetic relationships within marsupials were investigated based on a 20.1-kilobase molecular supermatrix comprising 7 nuclear and 15 mitochondrial genes analyzed using both maximum likelihood and Bayesian approaches and 3 different partitioning strategies. The study revealed that base composition bias in the 3rd codon positions of mitochondrial genes misled even the partitioned maximum-likelihood analyses, whereas Bayesian analyses were less affected. After correcting for base composition bias, monophyly of the currently recognized marsupial orders, of Australidelphia, and of a clade comprising Dasyuromorphia, Notoryctes,and Peramelemorphia, were supported strongly by both Bayesian posterior probabilities and maximum-likelihood bootstrap values. Monophyly of the Australasian marsupials, of Notoryctes þ Dasyuromorphia, and of Caenolestes þ Australidelphia were less well supported. Within Diprotodontia, Burramyidae þ Phalangeridae received relatively strong support. Divergence dates calculated using a Bayesian relaxed molecular clock and multiple age constraints suggested at least 3 independent dispersals of marsupials from North to South America during the Late Cretaceous or early Paleocene. Within the Australasian clade, the macropodine radiation, the divergence of phascogaline and dasyurine dasyurids, and the divergence of perameline and peroryctine peramelemorphians all coincided with periods of significant environmental change during the Miocene. An analysis of ‘‘unrepresented basal branch lengths’’ suggests that the fossil record is particularly poor for didelphids and most groups within the Australasian radiation. -

A Phylogeny and Timescale for Marsupial Evolution Based on Sequences for Five Nuclear Genes
J Mammal Evol DOI 10.1007/s10914-007-9062-6 ORIGINAL PAPER A Phylogeny and Timescale for Marsupial Evolution Based on Sequences for Five Nuclear Genes Robert W. Meredith & Michael Westerman & Judd A. Case & Mark S. Springer # Springer Science + Business Media, LLC 2007 Abstract Even though marsupials are taxonomically less diverse than placentals, they exhibit comparable morphological and ecological diversity. However, much of their fossil record is thought to be missing, particularly for the Australasian groups. The more than 330 living species of marsupials are grouped into three American (Didelphimorphia, Microbiotheria, and Paucituberculata) and four Australasian (Dasyuromorphia, Diprotodontia, Notoryctemorphia, and Peramelemorphia) orders. Interordinal relationships have been investigated using a wide range of methods that have often yielded contradictory results. Much of the controversy has focused on the placement of Dromiciops gliroides (Microbiotheria). Studies either support a sister-taxon relationship to a monophyletic Australasian clade or a nested position within the Australasian radiation. Familial relationships within the Diprotodontia have also proved difficult to resolve. Here, we examine higher-level marsupial relationships using a nuclear multigene molecular data set representing all living orders. Protein-coding portions of ApoB, BRCA1, IRBP, Rag1, and vWF were analyzed using maximum parsimony, maximum likelihood, and Bayesian methods. Two different Bayesian relaxed molecular clock methods were employed to construct a timescale for marsupial evolution and estimate the unrepresented basal branch length (UBBL). Maximum likelihood and Bayesian results suggest that the root of the marsupial tree is between Didelphimorphia and all other marsupials. All methods provide strong support for the monophyly of Australidelphia. Within Australidelphia, Dromiciops is the sister-taxon to a monophyletic Australasian clade. -

Australian Marsupial Species Identification
G Model FSIGSS-793; No. of Pages 2 Forensic Science International: Genetics Supplement Series xxx (2011) xxx–xxx Contents lists available at ScienceDirect Forensic Science International: Genetics Supplement Series jo urnal homepage: www.elsevier.com/locate/FSIGSS Australian marsupial species identification a, b,e c,d d d Linzi Wilson-Wilde *, Janette Norman , James Robertson , Stephen Sarre , Arthur Georges a ANZPAA National Institute of Forensic Science, Victoria, Australia b Museum Victoria, Victoria, Australia c Australian Federal Police, Australian Capital Territory, Australia d University of Canberra, Australian Capital Territory, Australia e Melbourne University, Victoria, Australia A R T I C L E I N F O A B S T R A C T Article history: Wildlife crime, the illegal trade in animals and animal products, is a growing concern and valued at up to Received 10 October 2011 US$20 billion globally per year. Australia is often targeted for its unique fauna, proximity to South East Accepted 10 October 2011 Asia and porous borders. Marsupials of the order Diprotodontia (including koala, wombats, possums, gliders, kangaroos) are sometimes targeted for their skin, meat and for the pet trade. However, species Keywords: identification for forensic purposes must be underpinned by robust phylogenetic information. A Species identification Diprotodont phylogeny containing a large number of taxa generated from nuclear and mitochondrial Forensic data has not yet been constructed. Here the mitochondrial (COI and ND2) and nuclear markers (APOB, DNA IRBP and GAPD) are combined to create a more robust phylogeny to underpin a species identification COI Barcoding method for the marsupial order Diprotodontia. Mitochondrial markers were combined with nuclear Diprotodontia markers to amplify 27 genera of Diprotodontia. -

A Species-Level Phylogenetic Supertree of Marsupials
J. Zool., Lond. (2004) 264, 11–31 C 2004 The Zoological Society of London Printed in the United Kingdom DOI:10.1017/S0952836904005539 A species-level phylogenetic supertree of marsupials Marcel Cardillo1,2*, Olaf R. P. Bininda-Emonds3, Elizabeth Boakes1,2 and Andy Purvis1 1 Department of Biological Sciences, Imperial College London, Silwood Park, Ascot SL5 7PY, U.K. 2 Institute of Zoology, Zoological Society of London, Regent’s Park, London NW1 4RY, U.K. 3 Lehrstuhl fur¨ Tierzucht, Technical University of Munich, Alte Akademie 12, 85354 Freising-Weihenstephan, Germany (Accepted 26 January 2004) Abstract Comparative studies require information on phylogenetic relationships, but complete species-level phylogenetic trees of large clades are difficult to produce. One solution is to combine algorithmically many small trees into a single, larger supertree. Here we present a virtually complete, species-level phylogeny of the marsupials (Mammalia: Metatheria), built by combining 158 phylogenetic estimates published since 1980, using matrix representation with parsimony. The supertree is well resolved overall (73.7%), although resolution varies across the tree, indicating variation both in the amount of phylogenetic information available for different taxa, and the degree of conflict among phylogenetic estimates. In particular, the supertree shows poor resolution within the American marsupial taxa, reflecting a relative lack of systematic effort compared to the Australasian taxa. There are also important differences in supertrees based on source phylogenies published before 1995 and those published more recently. The supertree can be viewed as a meta-analysis of marsupial phylogenetic studies, and should be useful as a framework for phylogenetically explicit comparative studies of marsupial evolution and ecology. -

Event Information
BLANCO FOOD & EVENTS: RESTAURANT & CATERING AWARDS FOR EXCELLENCE National Event Caterer of the Year 2008 South Australian Event Caterer of the Year 2003, 2004, 2005, 2007, 2008, 2009 ENQUIRIES T. +61 8 8230 1313 PO Box 2669 South Australian Caterer of the Year 2003 [email protected] F. +61 8 8132 0813 Kent Town South Australian Hall of Fame 2006, 2010 www.blancofood.com.au South Australia 5071 South Australian Sanctuary Adelaide Zoo – Venue Caterer of the Year 2010, 2011 EVENT INFORMATION PLANE TREE DRIVE, ADELAIDE, SOUTH AUSTRALIA A BLANCO FOOD & EVENTS VENUE www.blancofood.com.au | [email protected] | +61 8 8230 1313 WELCOME Thank you for considering the Sanctuary Adelaide Zoo and the award winning Blanco Food & Events Team for your upcoming event. Opened in 2010, the Sanctuary Adelaide Zoo is a state of the art function facility located on the first floor of the Adelaide Zoo’s new $30 million re-development. This development has coincided with the arrival of Wang Wang and Funi – the only two giant pandas in the southern hemisphere. Sanctuary Adelaide Zoo provides the latest technology within flexible meeting, exhibition or banquet space over looking the magnificent parklands of Adelaide’s CBD fringe. Managed by Blanco Food & Events, our professional service and depth of experience is reflected in our multiple awards including “The Best Achievement in Catering” at the 2011 Australian Event Awards. Blanco’s partnership with Adelaide Zoo provides you with amazing animal encounters at your event which are unequalled. Giant pandas, tiger feeding, animal handling, can provide a memorable experience at your event.