Rationale and Design of Khuzestan Vitamin D Deficiency Screening Program in Pregnancy: a Stratified Randomized Vitamin D Supplementation Controlled Trial
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Future Strategies for Promoting Tourism and Petroleum Heritage in Khuzestan Province, Iran
Future strategies for promoting tourism and petroleum heritage in Khuzestan Province, Iran Sahar Amirkhani, Neda Torabi Farsani and Homa Moazzen Jamshidi Abstract Sahar Amirkhani and Purpose – Industrial tourism not only strives to preserve industrial heritage, but can also be a strategy for being Neda Torabi Farsani are both familiar with the history of industry and attracting tourists to new destinations. This paper examines the issue of based at the Department of promoting petroleum industrial tourism in the case of Khuzestan, Iran. The research aims at determining Museum and Tourism, Art appropriate strategies for promoting petroleum industrial tourism. University of Isfahan, – Design/methodology/approach The data were analysed through a strengths, weaknesses, opportunities, Isfahan, Iran. and threats (SWOT) model. Homa Moazzen Jamshidi is Findings – The results revealed the competitive strategy as the best. Lastly, strategies such as: concentric based at the Department of diversification, joint venture strategy, conglomerate diversification and horizontal diversification were proposed Economics and Arts as key solutions. The results support the view that establishing an exploratory ecomuseum in the territory of Entrepreneurship, Art Khuzestan Province can be a suitable concentric diversification strategy towards petroleum industrial sustainable tourism in the future. University of Isfahan, Originality/value – The main originality of this paper includes linking tourism with the petroleum (oil and natural Isfahan, Iran. gas) industry -

EPPO Bulletin E-Mail to Hq@Eppo
Entomology and Applied Science Letters Volume 6, Issue 2, Page No: 13-19 Copyright CC BY-NC-ND 4.0 Available Online at: www.easletters.com ISSN No: 2349-2864 Descriptive- Analytical Evaluation of Scorpion Sting Incidence in Masjed- Soleyman County, Southwestern Iran Hamid Kassiri 1*, Iman Khodkar 2, Mnsour Yousefi 3, Niusha Kasiri 2, Masoud Lotfi4 1Department of Medical Entomology, School of Health, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. 2Student Research Committee, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran 3Communicable Diseases Department, Masjed-Soleyman County Health Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. 4Abdanan Health Center, Ilam University of Medical Sciences, Ilam, Iran. ABSTRACT Scorpions are dangerous for humans due to having deadly and toxic sting. Scorpion sting is a major public health challenge in many countries. The south and southwest of Iran with about 95% species of scorpions are the most heavily occupied regions in the country. Khuzestan Province is highlighted for its scorpions and scorpionism amongst the provinces of Iran. Khuzestan with 19 species of scorpions is one of the most important regions in terms of scorpionism problem in the southwestern Iran. Therefore, this study was conducted with the aim of survey epidemiology of scorpion sting in Masjed-Soleyman County from 2015 till 2017. This research is a descriptive - analytical study. All the scorpionism cases who were referred to the 22-Bahman Hospital of Masjed-Soleyman during the study period were included in this research. The required information was extracted from the patients’ recorded data in the hospital. Information for each case were recorded in a special checklist and imported into the computer for statistical analysis. -

Khuzestan Province, Iran
Journal of Hydraulic Structures J. Hydraul. Struct., 2019; 6(1): 90- 104 DOI: 10.22055/jhs.2020.32747.1133 The Impact of outlier detection to estimate groundwater fluctuations using GRACE satellite data; Case Study: Khuzestan Province, Iran Mohammad Mehdi Riyahi1* Mohammad Jafarpour1 Lotfollah Emadali2 Mohammad Ali Sharifi3 Abstract Groundwater aquifers are one of the most significant freshwater resources in the world. Hence, the monitoring of these resources is particularly important for available water resources planning. Piezometric wells have traditionally been used to monitor groundwater. This approach is costly and pointwise, which is not feasible for places with steep topography and mountainous areas. Nowadays, remote sensing techniques are widely used in various fields of engineering as appropriate alternatives to traditional methods. In water resources management, the Gravity Recovery and Climate Experiment (GRACE) satellites can monitor groundwater changes with acceptable accuracies. This paper applied the GRACE satellite data for a 40-month period to assess the variation of the groundwater level in Khuzestan province. The Global Land Data Assimilation System (GLDAS) model was used to counteract the soil moisture effect in final results. The observed data from piezometric wells were pre-processed to detect outliers using the Mahalanobis algorithm in Khuzestan province. At last, the outputs of GRACE were compared with these processed observed data. Despite the relatively small size of the area in question, the results indicated -
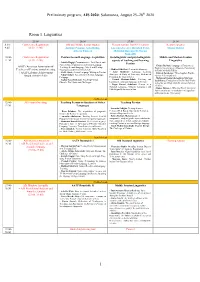
Preliminary Program, AIS 2020: Salamanca, August 25–28Th 2020
Preliminary program, AIS 2020: Salamanca, August 25–28th 2020 Room 1. Linguistics 25.08 26.08 27.08 28.08 8:30- Conference Registration Old and Middle Iranian studies Plenary session: Iran-EU relations Keynote speaker 9:45 (8:30–12:00) Antonio Panaino, Götz König, Luciano Zaccara, Rouzbeh Parsi, Maziar Bahari Alberto Cantera Mehrdad Boroujerdi, Narges Bajaoghli 10:00- Conference Registration Persian Second Language Acquisition Sociolinguistic and psycholinguistic Middle and Modern Iranian 11:30 (8:30–12:00) aspects of teaching and learning Linguistics - Latifeh Hagigi: Communicative, Task-Based, and Persian Content-Based Approaches to Persian Language - Chiara Barbati: Language of Paratexts as AATP (American Association of Teaching: Second Language, Mixed and Heritage Tool for Investigating a Monastic Community - Mahbod Ghaffari: Persian Interlanguage Teachers of Persian) annual meeting Classrooms at the University Level in Early Medieval Turfan - Azita Mokhtari: Language Learning + AATP Lifetime Achievement - Ali R. Abasi: Second Language Writing in Persian - Zohreh Zarshenas: Three Sogdian Words ( Strategies: A Study of University Students of (m and ryżי k .kי rγsי β יי Nahal Akbari: Assessment in Persian Language - Award (10:00–13:00) Persian in the United States Pedagogy - Mahmoud Jaafari-Dehaghi & Maryam - Pouneh Shabani-Jadidi: Teaching and - Asghar Seyed-Ghorab: Teaching Persian Izadi Parsa: Evaluation of the Prefixed Verbs learning the formulaic language in Persian Ghazals: The Merits and Challenges in the Ma’ani Kitab Allah Ta’ala -

Durham Research Online
Durham Research Online Deposited in DRO: 07 April 2008 Version of attached file: Published Version Peer-review status of attached file: Unknown Citation for published item: Seccombe, I. and Lawless, R. (1987) ’Work camps and company towns : settlement patterns and the Gulf oil industry.’, Working Paper. University of Durham, Centre for Middle Eastern and Islamic Studies, Durham. Further information on publisher’s website: http://www.dur.ac.uk/sgia/ Publisher’s copyright statement: Additional information: Use policy The full-text may be used and/or reproduced, and given to third parties in any format or medium, without prior permission or charge, for personal research or study, educational, or not-for-profit purposes provided that: • a full bibliographic reference is made to the original source • a link is made to the metadata record in DRO • the full-text is not changed in any way The full-text must not be sold in any format or medium without the formal permission of the copyright holders. Please consult the full DRO policy for further details. Durham University Library, Stockton Road, Durham DH1 3LY, United Kingdom Tel : +44 (0)191 334 3042 — Fax : +44 (0)191 334 2971 http://dro.dur.ac.uk UNIVERSITY OF DURHAM CENTRE FOR /dIDDLE EASTERN A NO ISLAMIC STUDIES Work Camps and Company Towns: Settlement Patterns and the Gulf Oil Industry r~~;':'SH UCR:. 'J,y~~< TS!. 'Ply CENTr.E : -'.';i.:t tf' I • .1 J. ~ .~ I ... Ian Seccomhe aod 'Rfclrard,L",Ies I ~ ... .. "_, _ •• ..I';;- vrj'.. ; 1""8'-.(:J -··•• , C"o_..... 1=5 Occasional Papers Series No. -

Durham Research Online
Durham Research Online Deposited in DRO: 07 April 2008 Version of attached le: Published Version Peer-review status of attached le: Unknown Citation for published item: Seccombe, I. and Lawless, R. (1987) 'Work camps and company towns : settlement patterns and the Gulf oil industry.', Working Paper. University of Durham, Centre for Middle Eastern and Islamic Studies, Durham. Further information on publisher's website: http://www.dur.ac.uk/sgia/ Publisher's copyright statement: Additional information: Use policy The full-text may be used and/or reproduced, and given to third parties in any format or medium, without prior permission or charge, for personal research or study, educational, or not-for-prot purposes provided that: • a full bibliographic reference is made to the original source • a link is made to the metadata record in DRO • the full-text is not changed in any way The full-text must not be sold in any format or medium without the formal permission of the copyright holders. Please consult the full DRO policy for further details. Durham University Library, Stockton Road, Durham DH1 3LY, United Kingdom Tel : +44 (0)191 334 3042 | Fax : +44 (0)191 334 2971 https://dro.dur.ac.uk UNIVERSITY OF DURHAM CENTRE FOR /dIDDLE EASTERN A NO ISLAMIC STUDIES Work Camps and Company Towns: Settlement Patterns and the Gulf Oil Industry r~~;':'SH UCR:. 'J,y~~< TS!. 'Ply CENTr.E : -'.';i.:t tf' I • .1 J. ~ .~ I ... Ian Seccomhe aod 'Rfclrard,L",Ies I ~ ... .. "_, _ •• ..I';;- vrj'.. ; 1""8'-.(:J -··•• , C"o_..... 1=5 Occasional Papers Series No. -

State and Tribes in Persia 1919-1925
State and Tribes in Persia 1919-1925 A case study On Political Role of the Great Tribes in Southern Persia Inauguraldissertation zur Erlangung des Grades eines Doktors der Philosophie (Dr. Phil.) Freie Universität Berlin Otto-Suhr-Institut für Politikwissenschaften Fachbereich Politik- und Sozialwissenschaften vorgelegt von: Javad Karandish Berlin, Januar 2003 Published 2011 1 1. Erstgutachter: Herr Prof. Dr. Wolf-Dieter Narr 2. Zweitgutachter: Herr Prof. Dr. Friedemann Büttner Disputationsdatum: 18.11.2003 2 PART I: GENERAL BACKGROUND ............................................................................................. 11 INTRODUCTION .................................................................................................................................... 12 1. THE STATEMENT OF A PROBLEM .......................................................................................... 12 1.1. Persia After the War.............................................................................................................. 15 2. THE RELEVANT QUESTIONINGS ............................................................................................. 16 3. THEORETICAL BASIS............................................................................................................. 17 4. THE METHOD OF RESEARCH .................................................................................................. 20 5. THE SUBJECT OF DISCUSSION ............................................................................................... -

Characteristics of Direct Human Impacts on the Rivers Karun and Dez in Lowland South-West Iran and Their Interactions with Earth Surface Movements
© 2016, Elsevier. Licensed under the Creative Commons Attribution-NonCommercial- NoDerivatives 4.0 International http://creativecommons.org/licenses/by-nc-nd/4.0/ Characteristics of direct human impacts on the rivers Karun and Dez in lowland south-west Iran and their interactions with earth surface movements Kevin P. Woodbridge, Daniel R. Parsons, Vanessa M. A. Heyvaert, Jan Walstra, Lynne E. Frostick Abstract Two of the primary external factors influencing the variability of major river systems, over river reach scales, are human activities and tectonics. Based on the rivers Karun and Dez in south-west Iran, this paper presents an analysis of the geomorphological responses of these major rivers to ancient human modifications and tectonics. Direct human modifications can be distinguished by both modern constructions and ancient remnants of former constructions that can leave a subtle legacy in a suite of river characteristics. For example, the ruins of major dams are characterised by a legacy of channel widening to 100's up to c. 1000 m within upstream zones that can stretch to channel distances of many kilometres upstream of former dam sites, whilst the legacy of major, ancient, anthropogenic river channel straightening can also be distinguished by very low channel sinuosities over long lengths of the river course. Tectonic movements in the region are mainly associated with young and emerging folds with NW–SE and N–S trends and with a long structural lineament oriented E–W. These earth surface movements can be shown to interact with both modern and ancient human impacts over similar timescales, with the types of modification and earth surface motion being distinguishable. -

Download the Entire 2015-2016 Annual Report In
THE ORIENTAL INSTITUTE 2015–2016 ANNUAL REPORT © 2016 by The University of Chicago. All rights reserved. Published 2016. Printed in the United States of America. The Oriental Institute, Chicago ISBN: 978-1-61491-035-0 Editor: Gil J. Stein Production facilitated by Emily Smith, Editorial Assistant, Publications Office Cover and overleaf illustration: Eastern stairway relief and columns of the Apadana at Persepolis. Herzfeld Expedition, 1933 (D. 13302) The pages that divide the sections of this year’s report feature images from the special exhibit “Persepolis: Images of an Empire,” on view in the Marshall and Doris Holleb Family Gallery for Special Exhibits, October 11, 2015, through September 3, 2017. See Ernst E. Herzfeld and Erich F. Schmidt, directors of the Oriental Institute’s archaeological expedition to Persepolis, on page 10. Printed by King Printing Company, Inc., Lowell, Massachusetts, USA CONTENTS CONTENTS INTRODUCTION INTRODUCTION. Gil J. Stein ........................................................ 5 IN MEMORIAM . 7 RESEARCH PROJECT REPORTS ÇADıR HÖYÜK . Gregory McMahon ............................................................ 13 CENTER FOR ANciENT MıDDLE EASTERN LANDSCAPES (CAMEL) . Emily Hammer ........................ 18 ChicAGO DEMOTic DicTıONARY (CDD) . Janet H. Johnson .......................................... 28 ChicAGO HıTTıTE AND ELECTRONic HıTTıTE DicTıONARY (CHD AND eCHD) . Theo van den Hout ........... 33 DENDARA . Gregory Marouard................................................................ 35 EASTERN -

An Analysis of Good Governance Role on Sustainable Tourism Revenues (Case: City of Masjedseleyman)
digitales archiv ZBW – Leibniz-Informationszentrum Wirtschaft ZBW – Leibniz Information Centre for Economics Shamai, Ali Article An analysis of good governance role on sustainable tourism revenues (case: city of MasjedSeleyman) Provided in Cooperation with: Iran Urban Economics Scientific Association, Tehran This Version is available at: http://hdl.handle.net/11159/2775 Kontakt/Contact ZBW – Leibniz-Informationszentrum Wirtschaft/Leibniz Information Centre for Economics Düsternbrooker Weg 120 24105 Kiel (Germany) E-Mail: [email protected] https://www.zbw.eu/econis-archiv/ Standard-Nutzungsbedingungen: Terms of use: Dieses Dokument darf zu eigenen wissenschaftlichen Zwecken This document may be saved and copied for your personal und zum Privatgebrauch gespeichert und kopiert werden. Sie and scholarly purposes. You are not to copy it for public or dürfen dieses Dokument nicht für öffentliche oder kommerzielle commercial purposes, to exhibit the document in public, to Zwecke vervielfältigen, öffentlich ausstellen, aufführen, vertreiben perform, distribute or otherwise use the document in public. If oder anderweitig nutzen. Sofern für das Dokument eine Open- the document is made available under a Creative Commons Content-Lizenz verwendet wurde, so gelten abweichend von diesen Licence you may exercise further usage rights as specified in Nutzungsbedingungen die in der Lizenz gewährten Nutzungsrechte. the licence. https://creativecommons.org/licenses/by-nc/3.0/ Leibniz-Informationszentrum Wirtschaft zbw Leibniz Information Centre for Economics To -

The Economic Geology of Iran Mineral Deposits and Natural Resources Springer Geology
Springer Geology Mansour Ghorbani The Economic Geology of Iran Mineral Deposits and Natural Resources Springer Geology For further volumes: http://www.springer.com/series/10172 Mansour Ghorbani The Economic Geology of Iran Mineral Deposits and Natural Resources Mansour Ghorbani Faculty of Geoscience Shahid Beheshti University Tehran , Iran ISBN 978-94-007-5624-3 ISBN 978-94-007-5625-0 (eBook) DOI 10.1007/978-94-007-5625-0 Springer Dordrecht Heidelberg New York London Library of Congress Control Number: 2012951116 © Springer Science+Business Media Dordrecht 2013 This work is subject to copyright. All rights are reserved by the Publisher, whether the whole or part of the material is concerned, speci fi cally the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on micro fi lms or in any other physical way, and transmission or information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed. Exempted from this legal reservation are brief excerpts in connection with reviews or scholarly analysis or material supplied speci fi cally for the purpose of being entered and executed on a computer system, for exclusive use by the purchaser of the work. Duplication of this publication or parts thereof is permitted only under the provisions of the Copyright Law of the Publisher’s location, in its current version, and permission for use must always be obtained from Springer. Permissions for use may be obtained through RightsLink at the Copyright Clearance Center. Violations are liable to prosecution under the respective Copyright Law. The use of general descriptive names, registered names, trademarks, service marks, etc. -

Effects of Climate Variables on the Incidence of Scorpion Stings in Iran for Five Years
RESEARCH OPEN ACCESS ISSN 1678-9199 www.jvat.org Effects of climate variables on the incidence of scorpion stings in Iran for five years Ahmad Ghorbani1, Behzad Mansouri2, Masoumeh Baradaran1* 1Toxicology Research Center, Medical Basic Sciences Institute, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. 2Department of Statistics, Shahid Chamran University of Ahvaz, Ahvaz, Iran. Abstract Background: Although scorpionism is recorded worldwide, some regions such as Iran present a higher incidence. Due to the great prevalence of scorpion stings in Khuzestan province, southwestern Iran, the present study examined the relationship between different climate parameters and the scorpion sting rate in this area from April 2010 to March 2015. Methods: In this cross-sectional descriptive-analytical study, we considered all scorpion sting cases recorded in the Department of Infectious Diseases, Ahvaz Jundishapur University of Medical Sciences. Data were analyzed using statistics, frequency distribution Keywords: and Pearson’s correlation coefficient. Scorpion stings Results: A total of 104,197 cases of scorpion stings was recorded from 2010 to 2015. Climate factors The cumulative incidence of scorpion sting was 2.23%. The spatial distribution Scorpionism of scorpion stings showed that most cases occurred in the Dehdez district (4,504 Khuzestan province scorpion stings/100,000 inhabitants) and the Masjed Soleyman county (4,069 scorpion stings/100,000 inhabitants). A significant association was found between climate factors Iran (temperature, evaporation rate, sunshine duration, humidity, and precipitation) and the scorpion sting rate. An increase in rainfall and humidity coincided with a reduction in scorpion stings whereas an increase in temperature, evaporation, and sunshine duration was accompanied by a growth of scorpion stings.