Isolation of Bacteria and Fungi from Lake Vostok Accretion Ice
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Arctic and Antarctic Research Institute” Russian Antarctic Expedition
FEDERAL SERVICE OF RUSSIA FOR HYDROMETEOROLOGY AND ENVIRONMENTAL MONITORING State Institution “Arctic and Antarctic Research Institute” Russian Antarctic Expedition QUARTERLY BULLETIN ʋ2 (51) April - June 2010 STATE OF ANTARCTIC ENVIRONMENT Operational data of Russian Antarctic stations St. Petersburg 2010 FEDERAL SERVICE OF RUSSIA FOR HYDROMETEOROLOGY AND ENVIRONMENTAL MONITORING State Institution “Arctic and Antarctic Research Institute” Russian Antarctic Expedition QUARTERLY BULLETIN ʋ2 (51) April - June 2010 STATE OF ANTARCTIC ENVIRONMENT Operational data of Russian Antarctic stations Edited by V.V. Lukin St. Petersburg 2010 Editor-in-Chief - M.O. Krichak (Russian Antarctic Expedition –RAE) Authors and contributors Section 1 M. O. Krichak (RAE), Section 2 Ye. I. Aleksandrov (Department of Meteorology) Section 3 G. Ye. Ryabkov (Department of Long-Range Weather Forecasting) Section 4 A. I. Korotkov (Department of Ice Regime and Forecasting) Section 5 Ye. Ye. Sibir (Department of Meteorology) Section 6 I. V. Moskvin, Yu.G.Turbin (Department of Geophysics) Section 7 V. V. Lukin (RAE) Section 8 B. R. Mavlyudov (RAS IG) Section 9 V. L. Martyanov (RAE) Translated by I.I. Solovieva http://www.aari.aq/, Antarctic Research and Russian Antarctic Expedition, Reports and Glossaries, Quarterly Bulletin. Acknowledgements: Russian Antarctic Expedition is grateful to all AARI staff for participation and help in preparing this Bulletin. For more information about the contents of this publication, please, contact Arctic and Antarctic Research Institute of Roshydromet Russian Antarctic Expedition Bering St., 38, St. Petersburg 199397 Russia Phone: (812) 352 15 41; 337 31 04 Fax: (812) 337 31 86 E-mail: [email protected] CONTENTS PREFACE……………………….…………………………………….………………………….1 1. DATA OF AEROMETEOROLOGICAL OBSERVATIONS AT THE RUSSIAN ANTARCTIC STATIONS…………………………………….…………………………3 2. -

Office of Polar Programs
DEVELOPMENT AND IMPLEMENTATION OF SURFACE TRAVERSE CAPABILITIES IN ANTARCTICA COMPREHENSIVE ENVIRONMENTAL EVALUATION DRAFT (15 January 2004) FINAL (30 August 2004) National Science Foundation 4201 Wilson Boulevard Arlington, Virginia 22230 DEVELOPMENT AND IMPLEMENTATION OF SURFACE TRAVERSE CAPABILITIES IN ANTARCTICA FINAL COMPREHENSIVE ENVIRONMENTAL EVALUATION TABLE OF CONTENTS 1.0 INTRODUCTION....................................................................................................................1-1 1.1 Purpose.......................................................................................................................................1-1 1.2 Comprehensive Environmental Evaluation (CEE) Process .......................................................1-1 1.3 Document Organization .............................................................................................................1-2 2.0 BACKGROUND OF SURFACE TRAVERSES IN ANTARCTICA..................................2-1 2.1 Introduction ................................................................................................................................2-1 2.2 Re-supply Traverses...................................................................................................................2-1 2.3 Scientific Traverses and Surface-Based Surveys .......................................................................2-5 3.0 ALTERNATIVES ....................................................................................................................3-1 -

Antarctic Peninsula
Hucke-Gaete, R, Torres, D. & Vallejos, V. 1997c. Entanglement of Antarctic fur seals, Arctocephalus gazella, by marine debris at Cape Shirreff and San Telmo Islets, Livingston Island, Antarctica: 1998-1997. Serie Científica Instituto Antártico Chileno 47: 123-135. Hucke-Gaete, R., Osman, L.P., Moreno, C.A. & Torres, D. 2004. Examining natural population growth from near extinction: the case of the Antarctic fur seal at the South Shetlands, Antarctica. Polar Biology 27 (5): 304–311 Huckstadt, L., Costa, D. P., McDonald, B. I., Tremblay, Y., Crocker, D. E., Goebel, M. E. & Fedak, M. E. 2006. Habitat Selection and Foraging Behavior of Southern Elephant Seals in the Western Antarctic Peninsula. American Geophysical Union, Fall Meeting 2006, abstract #OS33A-1684. INACH (Instituto Antártico Chileno) 2010. Chilean Antarctic Program of Scientific Research 2009-2010. Chilean Antarctic Institute Research Projects Department. Santiago, Chile. Kawaguchi, S., Nicol, S., Taki, K. & Naganobu, M. 2006. Fishing ground selection in the Antarctic krill fishery: Trends in patterns across years, seasons and nations. CCAMLR Science, 13: 117–141. Krause, D. J., Goebel, M. E., Marshall, G. J., & Abernathy, K. (2015). Novel foraging strategies observed in a growing leopard seal (Hydrurga leptonyx) population at Livingston Island, Antarctic Peninsula. Animal Biotelemetry, 3:24. Krause, D.J., Goebel, M.E., Marshall. G.J. & Abernathy, K. In Press. Summer diving and haul-out behavior of leopard seals (Hydrurga leptonyx) near mesopredator breeding colonies at Livingston Island, Antarctic Peninsula. Marine Mammal Science.Leppe, M., Fernandoy, F., Palma-Heldt, S. & Moisan, P 2004. Flora mesozoica en los depósitos morrénicos de cabo Shirreff, isla Livingston, Shetland del Sur, Península Antártica, in Actas del 10º Congreso Geológico Chileno. -

Rapid Cenozoic Glaciation of Antarctica Induced by Declining
letters to nature 17. Huang, Y. et al. Logic gates and computation from assembled nanowire building blocks. Science 294, Early Cretaceous6, yet is thought to have remained mostly ice-free, 1313–1317 (2001). 18. Chen, C.-L. Elements of Optoelectronics and Fiber Optics (Irwin, Chicago, 1996). vegetated, and with mean annual temperatures well above freezing 4,7 19. Wang, J., Gudiksen, M. S., Duan, X., Cui, Y. & Lieber, C. M. Highly polarized photoluminescence and until the Eocene/Oligocene boundary . Evidence for cooling and polarization sensitive photodetectors from single indium phosphide nanowires. Science 293, the sudden growth of an East Antarctic Ice Sheet (EAIS) comes 1455–1457 (2001). from marine records (refs 1–3), in which the gradual cooling from 20. Bagnall, D. M., Ullrich, B., Sakai, H. & Segawa, Y. Micro-cavity lasing of optically excited CdS thin films at room temperature. J. Cryst. Growth. 214/215, 1015–1018 (2000). the presumably ice-free warmth of the Early Tertiary to the cold 21. Bagnell, D. M., Ullrich, B., Qiu, X. G., Segawa, Y. & Sakai, H. Microcavity lasing of optically excited ‘icehouse’ of the Late Cenozoic is punctuated by a sudden .1.0‰ cadmium sulphide thin films at room temperature. Opt. Lett. 24, 1278–1280 (1999). rise in benthic d18O values at ,34 million years (Myr). More direct 22. Huang, Y., Duan, X., Cui, Y. & Lieber, C. M. GaN nanowire nanodevices. Nano Lett. 2, 101–104 (2002). evidence of cooling and glaciation near the Eocene/Oligocene 8 23. Gudiksen, G. S., Lauhon, L. J., Wang, J., Smith, D. & Lieber, C. M. Growth of nanowire superlattice boundary is provided by drilling on the East Antarctic margin , structures for nanoscale photonics and electronics. -

The Evolution of the Antarctic Ice Sheet at the Eocene-Oligocene Transition
Geophysical Research Abstracts Vol. 19, EGU2017-8151, 2017 EGU General Assembly 2017 © Author(s) 2017. CC Attribution 3.0 License. The evolution of the Antarctic ice sheet at the Eocene-Oligocene Transition. Jean-Baptiste Ladant (1), Yannick Donnadieu (1,2), and Christophe Dumas (1) (1) LSCE, CNRS-CEA, Paris, France ([email protected]), (2) CEREGE, CNRS, Aix-en-Provence, France An increasing number of studies suggest that the Middle to Late Eocene has witnessed the waxing and waning of relatively small ephemeral ice sheets. These alternating episodes culminated in the Eocene-Oligocene transition (34 – 33.5 Ma) during which a sudden and massive glaciation occurred over Antarctica. Data studies have demonstrated that this glacial event is constituted of two 50 kyr-long steps, the first of modest (10 – 30 m of equivalent sea level) and the second of major (50 – 90 m esl) glacial amplitude, and separated by ∼ 200 kyrs. Since a decade, modeling studies have put forward the primary role of CO2 in the initiation of this glaciation, in doing so marginalizing the original “gateway hypothesis”. Here, we investigate the impacts of CO2 and orbital parameters on the evolution of the ice sheet during the 500 kyrs of the EO transition using a tri-dimensional interpolation method. The latter allows precise orbital variations, CO2 evolution and ice sheet feedbacks (including the albedo) to be accounted for. Our results show that orbital variations are instrumental in initiating the first step of the EO glaciation but that the primary driver of the major second step is the atmospheric pCO2 crossing a modelled glacial threshold of ∼ 900 ppm. -
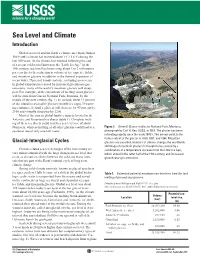
Sea Level and Climate Introduction
Sea Level and Climate Introduction Global sea level and the Earth’s climate are closely linked. The Earth’s climate has warmed about 1°C (1.8°F) during the last 100 years. As the climate has warmed following the end of a recent cold period known as the “Little Ice Age” in the 19th century, sea level has been rising about 1 to 2 millimeters per year due to the reduction in volume of ice caps, ice fields, and mountain glaciers in addition to the thermal expansion of ocean water. If present trends continue, including an increase in global temperatures caused by increased greenhouse-gas emissions, many of the world’s mountain glaciers will disap- pear. For example, at the current rate of melting, most glaciers will be gone from Glacier National Park, Montana, by the middle of the next century (fig. 1). In Iceland, about 11 percent of the island is covered by glaciers (mostly ice caps). If warm- ing continues, Iceland’s glaciers will decrease by 40 percent by 2100 and virtually disappear by 2200. Most of the current global land ice mass is located in the Antarctic and Greenland ice sheets (table 1). Complete melt- ing of these ice sheets could lead to a sea-level rise of about 80 meters, whereas melting of all other glaciers could lead to a Figure 1. Grinnell Glacier in Glacier National Park, Montana; sea-level rise of only one-half meter. photograph by Carl H. Key, USGS, in 1981. The glacier has been retreating rapidly since the early 1900’s. -

A Review of Ice-Sheet Dynamics in the Pine Island Glacier Basin, West Antarctica: Hypotheses of Instability Vs
Pine Island Glacier Review 5 July 1999 N:\PIGars-13.wp6 A review of ice-sheet dynamics in the Pine Island Glacier basin, West Antarctica: hypotheses of instability vs. observations of change. David G. Vaughan, Hugh F. J. Corr, Andrew M. Smith, Adrian Jenkins British Antarctic Survey, Natural Environment Research Council Charles R. Bentley, Mark D. Stenoien University of Wisconsin Stanley S. Jacobs Lamont-Doherty Earth Observatory of Columbia University Thomas B. Kellogg University of Maine Eric Rignot Jet Propulsion Laboratories, National Aeronautical and Space Administration Baerbel K. Lucchitta U.S. Geological Survey 1 Pine Island Glacier Review 5 July 1999 N:\PIGars-13.wp6 Abstract The Pine Island Glacier ice-drainage basin has often been cited as the part of the West Antarctic ice sheet most prone to substantial retreat on human time-scales. Here we review the literature and present new analyses showing that this ice-drainage basin is glaciologically unusual, in particular; due to high precipitation rates near the coast Pine Island Glacier basin has the second highest balance flux of any extant ice stream or glacier; tributary ice streams flow at intermediate velocities through the interior of the basin and have no clear onset regions; the tributaries coalesce to form Pine Island Glacier which has characteristics of outlet glaciers (e.g. high driving stress) and of ice streams (e.g. shear margins bordering slow-moving ice); the glacier flows across a complex grounding zone into an ice shelf coming into contact with warm Circumpolar Deep Water which fuels the highest basal melt-rates yet measured beneath an ice shelf; the ice front position may have retreated within the past few millennia but during the last few decades it appears to have shifted around a mean position. -

Download Preprint
Ross and Siegert: Lake Ellsworth englacial layers and basal melting 1 1 THIS IS AN EARTHARXIV PREPRINT OF AN ARTICLE SUBMITTED FOR 2 PUBLICATION TO THE ANNALS OF GLACIOLOGY 3 Basal melt over Subglacial Lake Ellsworth and it catchment: insights from englacial layering 1 2 4 Ross, N. , Siegert, M. , 1 5 School of Geography, Politics and Sociology, Newcastle University, Newcastle upon Tyne, 6 UK 2 7 Grantham Institute, Imperial College London, London, UK Annals of Glaciology 61(81) 2019 2 8 Basal melting over Subglacial Lake Ellsworth and its 9 catchment: insights from englacial layering 1 2 10 Neil ROSS, Martin SIEGERT, 1 11 School of Geography, Politics and Sociology, Newcastle University, Newcastle upon Tyne, UK 2 12 Grantham Institute, Imperial College London, London, UK 13 Correspondence: Neil Ross <[email protected]> 14 ABSTRACT. Deep-water ‘stable’ subglacial lakes likely contain microbial life 15 adapted in isolation to extreme environmental conditions. How water is sup- 16 plied into a subglacial lake, and how water outflows, is important for under- 17 standing these conditions. Isochronal radio-echo layers have been used to infer 18 where melting occurs above Lake Vostok and Lake Concordia in East Antarc- 19 tica but have not been used more widely. We examine englacial layers above 20 and around Lake Ellsworth, West Antarctica, to establish where the ice sheet 21 is ‘drawn down’ towards the bed and, thus, experiences melting. Layer draw- 22 down is focused over and around the NW parts of the lake as ice, flowing 23 obliquely to the lake axis, becomes afloat. -

Antarctic Primer
Antarctic Primer By Nigel Sitwell, Tom Ritchie & Gary Miller By Nigel Sitwell, Tom Ritchie & Gary Miller Designed by: Olivia Young, Aurora Expeditions October 2018 Cover image © I.Tortosa Morgan Suite 12, Level 2 35 Buckingham Street Surry Hills, Sydney NSW 2010, Australia To anyone who goes to the Antarctic, there is a tremendous appeal, an unparalleled combination of grandeur, beauty, vastness, loneliness, and malevolence —all of which sound terribly melodramatic — but which truly convey the actual feeling of Antarctica. Where else in the world are all of these descriptions really true? —Captain T.L.M. Sunter, ‘The Antarctic Century Newsletter ANTARCTIC PRIMER 2018 | 3 CONTENTS I. CONSERVING ANTARCTICA Guidance for Visitors to the Antarctic Antarctica’s Historic Heritage South Georgia Biosecurity II. THE PHYSICAL ENVIRONMENT Antarctica The Southern Ocean The Continent Climate Atmospheric Phenomena The Ozone Hole Climate Change Sea Ice The Antarctic Ice Cap Icebergs A Short Glossary of Ice Terms III. THE BIOLOGICAL ENVIRONMENT Life in Antarctica Adapting to the Cold The Kingdom of Krill IV. THE WILDLIFE Antarctic Squids Antarctic Fishes Antarctic Birds Antarctic Seals Antarctic Whales 4 AURORA EXPEDITIONS | Pioneering expedition travel to the heart of nature. CONTENTS V. EXPLORERS AND SCIENTISTS The Exploration of Antarctica The Antarctic Treaty VI. PLACES YOU MAY VISIT South Shetland Islands Antarctic Peninsula Weddell Sea South Orkney Islands South Georgia The Falkland Islands South Sandwich Islands The Historic Ross Sea Sector Commonwealth Bay VII. FURTHER READING VIII. WILDLIFE CHECKLISTS ANTARCTIC PRIMER 2018 | 5 Adélie penguins in the Antarctic Peninsula I. CONSERVING ANTARCTICA Antarctica is the largest wilderness area on earth, a place that must be preserved in its present, virtually pristine state. -

Antarctic Climate and Sea Ice Variability – a Brief Review Marilyn Raphael UCLA Geography
Antarctic Climate and Sea Ice Variability – a Brief Review Marilyn Raphael UCLA Geography WRCP Workshop on Seasonal to Multi- Decadal Predictability of Polar Climate Mean annual precipitation produced by NCEP2 for the years 1979–99 (mm yr21water equivalent). Bromwich et al, 2004 A significant upward trend 11.3 to 11.7 mm yr22 for 1979–99 is found from retrieved and forecast Antarctic precipitation over the continent. (a) Monthly and (b) annual time series for the modeled precipitation over all of Antarctica. Bromwich et al, 2004 Spatial pattern of temperature trends (degrees Celsius per decade) from reconstruction using infrared (TIR) satellite data. a, Mean annual trends for 1957–2006; b, Mean annual trends for 1969–2000, c–f, Seasonal trends for 1957–2006: winter (June, July, August; c); spring (September, October, November; d); summer (December, January, February; e); autumn (March, April, May; f). Over the long term (150 years) Antarctica has been warming, recent cooling trends in the 1990s attributed to positive trend in the SAM offset this warming. (Schneider et al, 2006) – ice cores Warming of Antarctica extends beyond the Antarctic Peninsula includes most of west Antarctica. Except in autumn, warming is apparent across most of the continent but is significant only over west Antarctica including the Peninsula (Steig et al, 2009). 1957 – 2006 reconstruction from satellite data. Steig et al, 2009 Steig et al, 2009 – reconstruction based on satellite and station data annual warming of 0.18C per decade for 1957 – 2006; winter and spring leading. Chapman and Walsh, 2007 – reconstruction based on station data and oceanic records 1950-2002: warming across most of West Antarctica Monaghan et al, 2009 – reconstruction 190-2005: warming across West Antarctica in all seasons; significant in spring and summer Trends are strongly seasonal. -

In the Lands of the Romanovs: an Annotated Bibliography of First-Hand English-Language Accounts of the Russian Empire
ANTHONY CROSS In the Lands of the Romanovs An Annotated Bibliography of First-hand English-language Accounts of The Russian Empire (1613-1917) OpenBook Publishers To access digital resources including: blog posts videos online appendices and to purchase copies of this book in: hardback paperback ebook editions Go to: https://www.openbookpublishers.com/product/268 Open Book Publishers is a non-profit independent initiative. We rely on sales and donations to continue publishing high-quality academic works. In the Lands of the Romanovs An Annotated Bibliography of First-hand English-language Accounts of the Russian Empire (1613-1917) Anthony Cross http://www.openbookpublishers.com © 2014 Anthony Cross The text of this book is licensed under a Creative Commons Attribution 4.0 International license (CC BY 4.0). This license allows you to share, copy, distribute and transmit the text; to adapt it and to make commercial use of it providing that attribution is made to the author (but not in any way that suggests that he endorses you or your use of the work). Attribution should include the following information: Cross, Anthony, In the Land of the Romanovs: An Annotated Bibliography of First-hand English-language Accounts of the Russian Empire (1613-1917), Cambridge, UK: Open Book Publishers, 2014. http://dx.doi.org/10.11647/ OBP.0042 Please see the list of illustrations for attribution relating to individual images. Every effort has been made to identify and contact copyright holders and any omissions or errors will be corrected if notification is made to the publisher. As for the rights of the images from Wikimedia Commons, please refer to the Wikimedia website (for each image, the link to the relevant page can be found in the list of illustrations). -

Ice Production in Ross Ice Shelf Polynyas During 2017–2018 from Sentinel–1 SAR Images
remote sensing Article Ice Production in Ross Ice Shelf Polynyas during 2017–2018 from Sentinel–1 SAR Images Liyun Dai 1,2, Hongjie Xie 2,3,* , Stephen F. Ackley 2,3 and Alberto M. Mestas-Nuñez 2,3 1 Key Laboratory of Remote Sensing of Gansu Province, Heihe Remote Sensing Experimental Research Station, Cold and Arid Regions Environmental and Engineering Research Institute, Chinese Academy of Sciences, Lanzhou 730000, China; [email protected] 2 Laboratory for Remote Sensing and Geoinformatics, Department of Geological Sciences, University of Texas at San Antonio, San Antonio, TX 78249, USA; [email protected] (S.F.A.); [email protected] (A.M.M.-N.) 3 Center for Advanced Measurements in Extreme Environments, University of Texas at San Antonio, San Antonio, TX 78249, USA * Correspondence: [email protected]; Tel.: +1-210-4585445 Received: 21 April 2020; Accepted: 5 May 2020; Published: 7 May 2020 Abstract: High sea ice production (SIP) generates high-salinity water, thus, influencing the global thermohaline circulation. Estimation from passive microwave data and heat flux models have indicated that the Ross Ice Shelf polynya (RISP) may be the highest SIP region in the Southern Oceans. However, the coarse spatial resolution of passive microwave data limited the accuracy of these estimates. The Sentinel-1 Synthetic Aperture Radar dataset with high spatial and temporal resolution provides an unprecedented opportunity to more accurately distinguish both polynya area/extent and occurrence. In this study, the SIPs of RISP and McMurdo Sound polynya (MSP) from 1 March–30 November 2017 and 2018 are calculated based on Sentinel-1 SAR data (for area/extent) and AMSR2 data (for ice thickness).