Antioxidant Activity of Myrtus Communis L. and Myrtus Nivellei Batt. & Trab. Extracts: a Brief Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
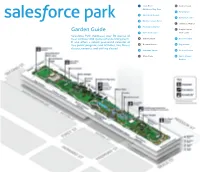
Salesforce Park Garden Guide
Start Here! D Central Lawn Children’s Play Area Garden Guide6 Palm Garden 1 Australian Garden Start Here! D Central Lawn Salesforce Park showcases7 California over Garden 50 species of Children’s Play Area 2 Mediterraneantrees and Basin over 230 species of understory plants. 6 Palm Garden -ã ¼ÜÊ ÊăØÜ ØÊèÜãE úØƀØÊèÃJapanese Maples ¼ÃØ Ê¢ 1 Australian Garden 3 Prehistoric¢ØÕ輫ÕØÊ£ØÂÜÃã«ó«ã«Üŧ¼«¹ĆãÃÜÜ Garden 7 California Garden ¼ÜÜÜŧÊÃØãÜŧÃØ¢ã«Ã£¼ÜÜÜũF Amphitheater Garden Guide 2 Mediterranean Basin 4 Wetland Garden Main Lawn E Japanese Maples Salesforce Park showcases over 50 species of 3 Prehistoric Garden trees and over 230 species of understory plants. A Oak Meadow 8 Desert Garden F Amphitheater It also offers a robust year-round calendar of 4 Wetland Garden Main Lawn free public programs and activities, like fitness B Bamboo Grove 9 Fog Garden Desert Garden classes, concerts, and crafting classes! A Oak Meadow 8 5 Redwood Forest 10 Chilean Garden B Bamboo Grove 9 Fog Garden C Main Plaza 11 South African 10 Chilean Garden Garden 5 Redwood Forest C Main Plaza 11 South African Garden 1 Children’s Australian Play Area Garden ABOUT THE GARDENS The botanist aboard the Endeavor, Sir Joseph Banks, is credited with introducing many plants from Australia to the western world, and many This 5.4 acre park has a layered soil system that plants today bear his name. balances seismic shifting, collects and filters storm- water, and irrigates the gardens. Additionally, the soil Native to eastern Australia, Grass Trees may grow build-up and dense planting help offset the urban only 3 feet in 100 years, and mature plants can be heat island effect by lowering the air temperature. -

Structures and Bioactive Properties of Myrtucommulones and Related Acylphloroglucinols from Myrtaceae
molecules Review Structures and Bioactive Properties of Myrtucommulones and Related Acylphloroglucinols from Myrtaceae Rosario Nicoletti 1,2 , Maria Michela Salvatore 3 , Pasquale Ferranti 2 and Anna Andolfi 3,* 1 Council for Agricultural Research and Economics, Research Centre for Olive, Citrus and Tree Fruit, 81100 Caserta, Italy; [email protected] 2 Department of Agriculture, University of Naples ‘Federico II’, 80055 Portici, Italy; [email protected] 3 Department of Chemical Sciences, University of Naples ‘Federico II’, 80126 Naples, Italy; [email protected] * Correspondence: andolfi@unina.it; Tel.: +39-081-2539179 Academic Editors: Francesco Vinale and Maria Luisa Balestrieri Received: 2 December 2018; Accepted: 17 December 2018; Published: 19 December 2018 Abstract: Myrtaceae are a group of plants that include a number of renowned species used in ethnomedicine in many areas worldwide. Their valuable therapeutic properties have stimulated a fruitful research activity addressed to the identification of the bioactive components of their extracts yielding a great diversity of terpenes; polyphenols; and other exclusive products. Among the latter, starting with the discovery of myrtucommulone A from myrtle (Myrtus communis), a series of structurally-related acylphloroglucinol compounds have been characterized from several species that represent the basic active principles to be considered in view of possible drug development. Aspects concerning chemical and biological properties of these products are reviewed in the present paper. Keywords: myrtucommulone; acylphloroglucinols; Myrtaceae; plant extracts; biological activities 1. Introduction Myrtle (Myrtus communis) is a typical shrub of maquis and coastal bushes native of the Mediterranean area and Western Asia. It is well-known in traditional medicine, and for centuries its leaves and berries have found ethnomedical application in the treatment of several disorders of the digestive apparatus, as well as pulmonary and skin diseases [1,2]. -

Identification of Myrtle Rust (Uredo Rangelii)
Identification of Myrtle Rust (Uredo rangelii) 6 October 2010 Hosts Myrtle Rust has been found on the NSW Central Coast on eleven species of cultivated native plants: • Agonis flexuosa (willow myrtle) cv. 'Afterdark' and cv. 'Burgundy' • Tristania neriifolia (water gum) • Syncarpia glomulifera (turpentine) • Callistemon viminalis (bottle brush) • Leptospermum rotundifolium (tea tree) • Syzygium leumannii x Syzygium wilsonii (lilly pilly) • Syzygium jambos (rose apple) • Syzygium australe cv. 'Meridian Midget' • Metrosideros collina cv. Dwarf • Austromyrtus inophloia cv. 'Aurora' and 'Blushing Beauty' (renamed to Gossia inophloia) • Rhodamnia rubescens (scrub turpentine) Other known hosts include Myrtus communis (common myrtle). At present, severe infestation has only been observed on A. flexuosa (willow myrtle) cv. 'Afterdark', Tristania neriifolia (water gum) and Austromyrtus inophloia cv. 'Aurora' and 'Blushing Beauty'. Spread Rust spores travel very long distances on the wind and may infect stands of susceptible plants many kilometres from the original infestation. Rust spores are also gathered and spread by bees. These are natural means of spread that are difficult to control. Humans can also easily spread Myrtle Rust in infested plant material including cut flowers and nursery stock, on clothing and dirty equipment including containers and pruning shears, and on contaminated timber products. Always practise good hygiene when working with native plants and general nursery stock. Images See the following pages. Reporting To report suspect cases of Myrtle Rust, please call the Exotic Plant Pest Hotline on 1800 084 881. © State of New South Wales through Department of Industry and Investment (Industry & Investment NSW) 2010. You may copy, distribute and otherwise freely deal with this publication for any purpose, provided that you attribute Industry & Investment NSW as the owner. -

The Forgotten Myrtle of the Alhambra Gardens of Granada: Restoring and Authenticating World Heritage
J. Agr. Sci. Tech. (2016) Vol. 18: 1975-1983 RESEARCH NOTES The Forgotten Myrtle of the Alhambra Gardens of Granada: Restoring and Authenticating World Heritage R. De la Herrán 1, M. Casares 2, F. Robles 1, J. Tito 3, R. Navajas-Pérez 1, M. J. Molina- Luzón 1, M. de los Reyes Gonzalez-Tejero 2, P. J. Sola-Campoy 1, A. Gutiérrez-Guerrero 1, ∗ and J. C. Ruiz-Rejón 1 ABSTRACT In the Alhambra (Granada, Spain), and in other Moorish locations, several individuals of the original variety of myrtle, the emblematic plant of their gardens, have been identified and genetically authenticated. After microsatellite analysis, we differentiated between the wild form ( Myrtus communis L.) and two cultivated varieties: the one original to the Alhambra, the Moorish myrtle (subsp. baetica ), and the variety introduced in more modern times (subsp. tarentina ). The genetic and morphological differences between these two varieties confirm the taxonomic distinctness of the subsp. baetica . With very few individuals known, this Moorish myrtle is on the verge of extinction. The genetic identification offers the opportunity to restore a key element of this 14th-century garden and enhance the authenticity of a World Heritage site. Keywords : Alhambra, Microsatellite, Mirtus communis , Subspecies, Taxon. INTRODUCTION In 1943, the gardens of the Alhambra and the surrounding area of Generalife were The Alhambra, one of the largest designated as Historical Gardens, and in medieval complexes surviving in Europe, 1984 UNESCO declared them a World originally had a fortress, several palaces and Heritage site. In fact, these gardens may be an aristocratic quarter, surrounded by among the oldest in Europe and, since orchards and gardens. -

Effect of Myrtus Communis Essential Oil on the Mediterranean Fruit Fly Mating Behaviour
Biosciences, Biotechnology Research Asia Vol. 5(2), 537-540 (2008) Effect of Myrtus communis essential oil on the Mediterranean fruit fly mating behaviour SAMIA ELFEKIH* and M. A. ABDERRABBA Unité de recherche physico-chimique moléculaire, IPEST, BP 51, La Marsa 2070 Tunis (Tunisia). (Received: October 25, 2008; Accepted: December 17, 2008) ABSTRACT The Mediterranean fruit fly Ceratitis capitata (Wiedmann) is a key agricultural insect pest. It has a wide range of host plants especially citrus trees. Medfly males are strongly attracted to plant semiochemicals. Exposure to such substance increased their mating success. In the current study, it was investigated whether a similar effect resulted from male exposure to the myrtle (M. communis) essential oil. Myrtle is a perennial shrub belonging to the Myrtaceae family and is widely distributed in the Mediterranean area. Myrtle essential oil was extracted and analysed by GC and GC-MS. The chemical composition revealed that most of the major components are monoterpenes (1,8-Cineole, alpha-pinene, Linalool, Limonene) and are similary found in citrus flavedo and orange essential oil. Laboratory trials suggest that exposure to myrtle essential oil significantly enhanced the medfly mating behaviour. It can be inferred from these tests that the myrtle essential oil can be used in insect pest management strategies such as the Sterile Insect Technique (SIT), to improve the mating competitiveness of sterile mass-reared males compared to wild males and therefore contribute to significant reduction of the medfly populations in fruit orchards. Key words: Myrtus communis, monoterpenes, Ceratitis capitata, mating behaviour. INTRODUCTION activity in enhancing the medfly mating success. -

(12) United States Patent (10) Patent No.: US 9,017,738 B2 Efrahimi (45) Date of Patent: Apr
US009017738B2 (12) United States Patent (10) Patent No.: US 9,017,738 B2 Efrahimi (45) Date of Patent: Apr. 28, 2015 (54) COMPOSITION FORTREATING (56) References Cited HEMORRHODS U.S. PATENT DOCUMENTS (71) Applicant: Eyal Efrahimi, Jerusalem (IL) 201 1 O274772 A1 ck 1 1, 2011 Kucukay et al. 424,727 (72) Inventor: Eyal Efrahimi, Jerusalem (IL) FOREIGN PATENT DOCUMENTS (*) Notice: Subject to any disclaimer, the term of this IT 2002-MI2263 A1 * 4, 2004 patent is extended or adjusted under 35 U.S.C. 154(b) by 0 days. * cited by examiner (21) Appl. No.: 13/769,848 Primary Examiner — Susan Hoffman (74) Attorney, Agent, or Firm — Mark M. Friedman (22) Filed: Feb. 19, 2013 (57) ABSTRACT (65) Prior Publication Data - The composition for treating hemorrhoids includes dry com US 2014/O234453 A1 Aug. 21, 2014 ponents: oleander (nerium Oleander), myrtle (myrtus commu mis)and bay laurel (laurus nobilis), which are then mixed into (51) Int. Cl. a homogenous solution with olive oil. In the preferred A6 IK 36/63 (2006.01) embodiment, the composition for treating hemorrhoids A6 IK 36/6 (2006.01) includes the following components: 30%–70% by weight of A6 IK36/54 (2006.01) olive oil, 27%-44% by weight of oleander (nerium oleander), A6 IK 36/24 (2006.01) 15%-25% percentage by weight of myrtle (myrtus communis) (52) U.S. Cl. and 5%-15% percentage by weight of bay laurel (laurus CPC ................. A61K 36/63 (2013.01); A61 K36/54 nobilis). For the most preferred embodiment, for 1 liter (about (2013.01); A61K 36/61 (2013.01); A61K 36/24 1000 cubic centimeters) of olive oil, 1000 cubic centimeters (2013.01) (cc) of the dry components of the composition may include (58) Field of Classification Search 70% percentage by weight of oleander (nerium oleander), CPC ...... -

Plant and Landscape Guide Rancho Santa Fe, California, Is Considered to Be in a Very High Fire Hazard Severity Zone Because of Its Unique Characteristics
Plant and Landscape Guide Rancho Santa Fe, California, is considered to be in a very high fire hazard severity zone because of its unique characteristics. It is considered a Wildland Urban Interface area because of the proximity of the natural chaparral vegetation to developed areas, often immediately abutting structures. Additionally, warm coastal weather, Santa Ana winds, mountainous terrain, and steep slopes contribute to the very high fire hazard severity zone designation. DistrictIn an effort (RSFFPD) to protect does homes not allow from certain a future types devastating of trees, Wildlandplants, or fire shrubs such to as be the ones experienced in 2003 and 2007, the Rancho Santa Fe Fire Protection planted within certain distances of structures. This booklet contains valuable educateinformation the publicpertaining on RSFFPD’s to both desirable ordinances and regarding undesirable landscaping trees, shrubs, so they can ground covers, vines, roadway clearances, and palm trees. The goal is to Lady Bank’s Rose increase the the chances of their home surviving a wildfire. Please feel free to contactPlease Note: the Fire District if you have any questions, comments, or concerns. 1. THIS IS NOT A COMPREHENSIVE LIST. This booklet is intended to simply guide the public on what types of trees and shrubs are acceptable within the Fire District. Other trees and shrubs not listed 2. may also be acceptable upon approval by the RSFFPD. Trees listed as requiring 30-foot spacing from the drip line to the structure are considered non-fire resistive trees by the RSFFPD. Consult a design professional or the Fire District for site-specific 3. -

Mitteilungen Der Deutschen Dendrologischen Gesellschaft
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Mitteilungen der Deutschen Dendrologischen Gesellschaft Jahr/Year: 1913 Band/Volume: 22 Autor(en)/Author(s): Goeze E. Artikel/Article: Myrtaceen, Lauraceen, Oleaceen, Aurantiaceen. 236-254 236 ^^- E. Goeze: 19I3- Myrtaceen, Lauraceen, Oleaceen, Aurantiaceen. Eine pflanzengeographisch-kulturgeschichlliche Studie. Von Dr. E. Goeze, Berlin. Dank pflanzengeographischen Forschungen ist das Dunkel, welches über viele kulturgeschichtliche Daten ausgebreitet lag, mehr und mehr gelichtet, sind manche damit im Zusammenhange stehende, schon recht veraltete Irrtümer nach und nach beseitigt worden. An einzelnen Arten der vier obengenannten Familien, vorzugs- weise tropischen Florengebieten angehörend, ließe sich dies weiter nachweisen, und die in Kultus und Poesie vielgepriesene Myrte, der nicht minder berühmte Lor- beer, sodann auch der altehrwürdige Ölbaum, die in ihrer ursprünglichen Heimat seit undenklichen Zeiten hochgeschätzten Orangen-Limonen können sich noch jetzt wie einst im Altertum des Vorrechts rühmen, nicht nur unter ihren Stamm- verwandten , sondern im gesamten Pflanzenreiche eine bevorzugte Sonderstellung einzunehmen. Wohl mögen die beiden erstgenannten in unserer übermodernen Zeit von ihrem dereinstigen Glänze manches eingebüßt haben, ihre traditionellen Reize auf Alt und Jung sind ihnen aber verblieben. Wenn man dann ferner auch zu dem praktischen Gesichtspunkte Stellung zu nehmen gesinnt -

Perennial Edible Fruits of the Tropics: an and Taxonomists Throughout the World Who Have Left Inventory
United States Department of Agriculture Perennial Edible Fruits Agricultural Research Service of the Tropics Agriculture Handbook No. 642 An Inventory t Abstract Acknowledgments Martin, Franklin W., Carl W. Cannpbell, Ruth M. Puberté. We owe first thanks to the botanists, horticulturists 1987 Perennial Edible Fruits of the Tropics: An and taxonomists throughout the world who have left Inventory. U.S. Department of Agriculture, written records of the fruits they encountered. Agriculture Handbook No. 642, 252 p., illus. Second, we thank Richard A. Hamilton, who read and The edible fruits of the Tropics are nnany in number, criticized the major part of the manuscript. His help varied in form, and irregular in distribution. They can be was invaluable. categorized as major or minor. Only about 300 Tropical fruits can be considered great. These are outstanding We also thank the many individuals who read, criti- in one or more of the following: Size, beauty, flavor, and cized, or contributed to various parts of the book. In nutritional value. In contrast are the more than 3,000 alphabetical order, they are Susan Abraham (Indian fruits that can be considered minor, limited severely by fruits), Herbert Barrett (citrus fruits), Jose Calzada one or more defects, such as very small size, poor taste Benza (fruits of Peru), Clarkson (South African fruits), or appeal, limited adaptability, or limited distribution. William 0. Cooper (citrus fruits), Derek Cormack The major fruits are not all well known. Some excellent (arrangements for review in Africa), Milton de Albu- fruits which rival the commercialized greatest are still querque (Brazilian fruits), Enriquito D. -

Pimenta Dioica
Pimenta dioica • Caryophyllus pimento Mill. • Eugenia divaricata var. ovalis O.Berg • Eugenia micrantha (Kunth) DC. • Eugenia micrantha Bertol., Fl. Guatimal., 22. 1840. • Eugenia pimenta (L.) DC. • Eugenia pimenta var. longifolia (Sims) DC. • Eugenia pimenta var. ovalifolia DC. • Evanesca crassifolia Raf. • Evanesca micrantha Bertol. • Myrtus aromatica Salisb., Prodr. Stirp. Chap. Allerton 354. 1796. • Myrtus pimenta L., Sp. Pl., 472. 1753. • Myrtus pimenta Ortega • Myrtus pimenta var. brevifolia Hayne Pimenta dioica • Myrtus pimenta var. longifolia Sims • Myrtus piperita Sessé & Moc. 1 Taxonavigation • Pimenta aromatica Kostel. Familia: Myrtaceae • Pimenta officinalis Lindl., Collect. Bot., sub t. 19. Subfamilia: Myrtoideae 1821. Tribus: Myrteae Genus: Pimenta • Pimenta officinalis O.Berg Species: Pimenta dioica • Pimenta officinalis var. longifolia (Sims) O.Berg 2 Name • Pimenta officinalis var. ovalifolia (DC.) O.Berg • Pimenta officinalis var. tenuifolia O. Berg Pimenta dioica (L.) Merr., Contr. Gray Herb. 165:37. 1947. • Pimenta pimenta (L.) Cockerell • Pimenta pimenta (L.) H. Karst., Deut. Fl., 790. 2.1 Synonyms 1882, nom. inadmiss. Basionym • Pimenta vulgaris Lindl. • Pimentus aromatica (Salisb.) Raf. Sylva Tellur. • Myrtus dioica L., Syst. nat. ed. vol. 10, 1056. 1759. 158. 1838. Heterotypic • Pimentus vera Raf. 1 2 4 VERNACULAR NAMES 3 References • Contributions from the Gray Herbarium of Harvard University. Cambridge, MA 165:37. 1947 • USDA, ARS, Germplasm Resources Information Network. Pimenta dioica in the Germplasm Re- sources Information -

UCC Library and UCC Researchers Have Made This Item Openly Available. Please Let Us Know How This Has Helped You. Thanks! Downlo
UCC Library and UCC researchers have made this item openly available. Please let us know how this has helped you. Thanks! Title Myrteae phylogeny, calibration, biogeography and diversification patterns: increased understanding in the most species rich tribe of Myrtaceae Author(s) Vasconcelos, Thais N. C.; Proença, Carol E. B.; Ahmad, Berhaman; Aguilar, Daniel S.; Aguilar, Reinaldo; Amorim, Bruno S.; Campbell, Keron; Costa, Itayguara R.; De-Carvalho, Plauto S.; Faria, Jair E. Q.; Giaretta, Augusto; Kooij, Pepijn W.; Lima, Duane F.; Mazine, Fiorella F.; Peguero, Brigido; Prenner, Gerhard; Santos, Matheus F.; Soewarto, Julia; Wingler, Astrid; Lucas, Eve J. Publication date 2017-01-06 Original citation Vasconcelos, T. N. C., Proença, C. E. B., Ahmad, B., Aguilar, D. S., Aguilar, R., Amorim, B. S., Campbell, K., Costa, I. R., De-Carvalho, P. S., Faria, J. E. Q., Giaretta, A., Kooij, P. W., Lima, D. F., Mazine, F. F., Peguero, B., Prenner, G., Santos, M. F., Soewarto, J., Wingler, A. and Lucas, E. J. (2017) ‘Myrteae phylogeny, calibration, biogeography and diversification patterns: increased understanding in the most species rich tribe of Myrtaceae’, Molecular Phylogenetics and Evolution, 109, pp. 113-137. doi:10.1016/j.ympev.2017.01.002 Type of publication Article (peer-reviewed) Link to publisher's http://dx.doi.org/10.1016/j.ympev.2017.01.002 version Access to the full text of the published version may require a subscription. Rights © 2017, Elsevier Inc. All rights reserved. This manuscript version is made available under the CC-BY-NC-ND 4.0 license https://creativecommons.org/licenses/by-nc-nd/4.0/ Embargo information Access to this article is restricted until 12 months after publication by request of the publisher. -

Plants for a 'Sustainable” -- Low Maintenance – Garden and Landscape in Arroyo Grande
PLANTS FOR A ‘SUSTAINABLE” -- LOW MAINTENANCE – GARDEN AND LANDSCAPE IN ARROYO GRANDE Low water use, minimal fertilizer needs, no special care Large Trees -- Cedrus libanii atlantica ‘Glauca’ BLUE ATLAS CEDAR Cedrus deodara DEODAR CEDAR Cinnamomum camphora CAMPHOR Gingko biloba GINGKO Pinus canariensis CANARY ISLAND PINE Pinus pinea ITALIAN STONE PINE Pinus sabiniana GRAY PINE Pinus torreyana TORREY PINE Quercus ilex HOLLY OAK Quercus suber CORK OAK Medium Trees -- Allocasuarina verticillata SHE-OAK Arbutus ‘Marina’ HYBRID STRAWBERRY TREE Brachychiton populneus KURRAJONG, AUSTRALIAN BOTTLE TREE Brahea armata BLUE HESPER PALM Butia capitata PINDO PALM Eucalyptus nicholii PEPPERMINT GUM Eucalyptus polyanthemos SILVER DOLLAR GUM Calocedrus decurrens INCENSE CEDAR Cupressus arizonica ARIZONA CYPRESS Cupressus forbesii TECATE CYPRESS Geijera parviflora AUSTRALIAN WILLOW Gleditsia triacanthos inermis THORNLESS HONEY LOCUST Juniperus scopulorum ‘Tolleson’s Blue Weeping’ BLUE WEEPING JUNIPER Melaleuca linariifolia FLAXLEAF PAPERBARK Metrosideros excelsus NEW ZEALAND CHRISTMAS TREE Olea europaea OLIVE (only fruitless cultivars such as ‘Majestic Beauty’, ‘Wilsoni’) Pinus halepensis ALEPPO PINE Pistacia chinensis CHINESE PISTACHE Quercus chrysolepis CANYON LIVE OAK Sequoiadendron giganteum GIANT REDWOOD © Copyright Joe Seals 2009 Small Trees Acacia baileyana BAILEY’S ACACIA Acacia pendula WEEPING MYALL Celtis australis EUROPEAN HACKBERRY x Chiltalpa tashkentensis CHILTALPA Cordyline australis CABBAGE PALM Cotinus coggygria SMOKE TREE Eucalyptus