CHAPTER 5: Bonding Theories - Explaining Molecular Geometry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Shapes and Structures of Organic Molecules HYBRIDISATION
Department of Chemistry Anugrah Memorial College, Gaya Class- B.Sc. 1 (Hons) Subject- Organic Chemistry Paper- 1C Unit- Shapes and Structures of Organic molecules Teacher – Dr. Nidhi Tripathi Assistant Professor Shapes and Structures of Organic molecules HYBRIDISATION The formation of bonds is no less than the act of courtship. Atoms come closer, attract to each other and gradually lose a little part of themselves to the other atoms. In chemistry, the study of bonding, that is, Hybridization is of prime importance. What happens to the atoms during bonding? What happens to the atomic orbitals? The answer lies in the concept of Hybridisation. Let us see! Introducing Hybridisation All elements around us, behave in strange yet surprising ways. The electronic configuration of these elements, along with their properties, is a unique concept to study and observe. Owing to the uniqueness of such properties and uses of an element, we are able to derive many practical applications of such elements. When it comes to the elements around us, we can observe a variety of physical properties that these elements display. The study of hybridization and how it allows the combination of various molecules in an interesting way is a very important study in science. Understanding the properties of hybridisation lets us dive into the realms of science in a way that is hard to grasp in one go but excellent to study once we get to know more about it. Let us get to know more about the process of hybridization, which will help us understand the properties of different elements. What is Hybridization? Scientist Pauling introduced the revolutionary concept of hybridization in the year 1931. -

No Rabbit Ears on Water. the Structure of the Water Molecule
No Rabbit Ears on Water The Structure of the Water Molecule: What Should We Tell the Students? Michael Laing University of Natal. Durban, South Africa In the vast majority of high school (I)and freshman uni- He also considers the possibility of s character in the bond- versity (2) chemistry textbooks, water is presented as a bent ing orbitals of OF2 and OH2 to improve the "strength" of the molecule whose bonding can be described bv the Gilles~ie- orbital (p 111) from 1.732 for purep to 2.00 for the ideal sp3. Nyholm approach with the oxygen atomsp3 Gyhridized. The Inclusion of s character in the hybrid will be "resisted in the lone uairs are said to he dominant causine the H-O-H anele case of atoms with an nnshared pair.. in the s orbital, to beieduced from the ideal 1091120to 104' and are regul&ly which is more stable than the p orbitals" (p 120). Estimates shown as equivalent in shape and size even being described of the H-E-H angle range from 91.2 for AsH3 to 93.5O for as "rabbit ears" (3). Chemists have even been advised to Hz0. He once again ascribes the anomalies for Hz0 to "re- wear Mickey Mouse ears when teaching the structure and uulsion of the charzes of the hvdroaeu atoms" (D 122). bonding of the H20molecule, presumably to emphasize the - In his famous hook (6)~itac~oro&kii describks the bond- shape of the molecule (and possibly the two equivalent lone ing in water and HzS (p 10). -

VSEPR Theory
VSEPR Theory The valence-shell electron-pair repulsion (VSEPR) model is often used in chemistry to predict the three dimensional arrangement, or the geometry, of molecules. This model predicts the shape of a molecule by taking into account the repulsion between electron pairs. This handout will discuss how to use the VSEPR model to predict electron and molecular geometry. Here are some definitions for terms that will be used throughout this handout: Electron Domain – The region in which electrons are most likely to be found (bonding and nonbonding). A lone pair, single, double, or triple bond represents one region of an electron domain. H2O has four domains: 2 single bonds and 2 nonbonding lone pairs. Electron Domain may also be referred to as the steric number. Nonbonding Pairs Bonding Pairs Electron domain geometry - The arrangement of electron domains surrounding the central atom of a molecule or ion. Molecular geometry - The arrangement of the atoms in a molecule (The nonbonding domains are not included in the description). Bond angles (BA) - The angle between two adjacent bonds in the same atom. The bond angles are affected by all electron domains, but they only describe the angle between bonding electrons. Lewis structure - A 2-dimensional drawing that shows the bonding of a molecule’s atoms as well as lone pairs of electrons that may exist in the molecule. Provided by VSEPR Theory The Academic Center for Excellence 1 April 2019 Octet Rule – Atoms will gain, lose, or share electrons to have a full outer shell consisting of 8 electrons. When drawing Lewis structures or molecules, each atom should have an octet. -

Structures and Properties of Substances
Structures and Properties of Substances Introducing Valence-Shell Electron- Pair Repulsion (VSEPR) Theory The VSEPR theory In 1957, the chemist Ronald Gillespie and Ronald Nyholm, developed a model for predicting the shape of molecules. This model is usually abbreviated to VSEPR (pronounced “vesper”) theory: Valence Shell Electron Pair Repulsion The fundamental principle of the VSEPR theory is that the bonding pairs (BP) and lone pairs (LP) of electrons in the valence level of an atom repel one another. Thus, the orbital for each electron pair is positioned as far from the other orbitals as possible in order to achieve the lowest possible unstable structure. The effect of this positioning minimizes the forces of repulsion between electron pairs. A The VSEPR theory The repulsion is greatest between lone pairs (LP-LP). Bonding pairs (BP) are more localized between the atomic nuclei, so they spread out less than lone pairs. Therefore, the BP-BP repulsions are smaller than the LP-LP repulsions. The repulsion between a bond pair and a lone-pair (BP-LP) is intermediate between the other two. In other words, in terms of decreasing repulsion: LP-LP > LP-BP > BP-BP The tetrahedral shape around a single-bonded carbon atom (e.g. in CH4), the planar shape around a carbon atom with two double bond (e.g. in CO2), and the bent shape around an oxygen atom in H2O result from repulsions between lone pairs and/or bonding pairs of electrons. The VSEPR theory The repulsion is greatest between lone pairs (LP-LP). Bonding pairs (BP) are more localized between the atomic nuclei, so they spread out less than lone pairs. -

Chemical Bonding II: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory Review Questions
Chemical Bonding II: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory Review Questions 10.1 J The properties of molecules are directly related to their shape. The sensation of taste, immune response, the sense of smell, and many types of drug action all depend on shape-specific interactions between molecules and proteins. According to VSEPR theory, the repulsion between electron groups on interior atoms of a molecule determines the geometry of the molecule. The five basic electron geometries are (1) Linear, which has two electron groups. (2) Trigonal planar, which has three electron groups. (3) Tetrahedral, which has four electron groups. (4) Trigonal bipyramid, which has five electron groups. (5) Octahedral, which has six electron groups. An electron group is defined as a lone pair of electrons, a single bond, a multiple bond, or even a single electron. H—C—H 109.5= ijj^^jl (a) Linear geometry \ \ (b) Trigonal planar geometry I Tetrahedral geometry I Equatorial chlorine Axial chlorine "P—Cl: \ Trigonal bipyramidal geometry 1 I Octahedral geometry I 369 370 Chapter 10 Chemical Bonding II The electron geometry is the geometrical arrangement of the electron groups around the central atom. The molecular geometry is the geometrical arrangement of the atoms around the central atom. The electron geometry and the molecular geometry are the same when every electron group bonds two atoms together. The presence of unbonded lone-pair electrons gives a different molecular geometry and electron geometry. (a) Four electron groups give tetrahedral electron geometry, while three bonding groups and one lone pair give a trigonal pyramidal molecular geometry. -

Orbital Hybridisation from Wikipedia, the Free Encyclopedia Jump To
Orbital hybridisation From Wikipedia, the free encyclopedia Jump to: navigation, search Four sp3 orbitals. Three sp2 orbitals. In chemistry, hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals suitable for the qualitative description of atomic bonding properties. Hybridised orbitals are very useful in the explanation of the shape of molecular orbitals for molecules. It is an integral part of valence bond theory. Although sometimes taught together with the valence shell electron-pair repulsion (VSEPR) theory, valence bond and hybridization are in fact not related to the VSEPR model.[1] The hybrids are named based on the atomic orbitals that are involved in the hybridization, and the geometries of the hybrids are also reflective of those of the atomic-orbital 3 contributors. For example, in methane (CH4) a set of sp orbitals are formed by mixing one s and three p orbitals on the carbon atom, and are directed towards the four hydrogen atoms which are located at the vertices of a regular tetrahedron. Contents [hide] • 1 Historical development • 2 Types of hybridisation 3 o 2.1 sp hybrids 2 o 2.2 sp hybrids o 2.3 sp hybrids • 3 Hybridisation and molecule shape o 3.1 Explanation of the shape of water o 3.2 Rationale for orbital exclusion in hypervalent molecules 3.2.1 d-orbitals in main group compounds 3.2.2 p-orbitals in transition metal complexes o 3.3 Description of bonding in hypervalent molecules 3.3.1 AX5 to AX7 main group compounds • 4 Hybridisation theory vs. MO theory • 5 See also • 6 References • 7 External links [edit] Historical development Chemist Linus Pauling first developed the hybridisation theory in order to explain the [2] structure of molecules such as methane (CH4). -

Molecular Shape
VSEPR Theory VSEPR Theory Shapes of Molecules Molecular Structure or Molecular Geometry The 3-dimensional arrangement of the atoms that make-up a molecule. Determines several properties of a substance, including: reactivity, polarity, phase of matter, color, magnetism, and biological activity. The chemical formula has no direct relationship with the shape of the molecule. VSEPR Theory Shapes of Molecules Molecular Structure or Molecular Geometry The 3-dimensional shapes of molecules can be predicted by their Lewis structures. Valence-shell electron pair repulsion (VSEPR) model or electron domain (ED) model: Used in predicting the shapes. The electron pairs occupy a certain domain. They move as far apart as possible. Lone pairs occupy additional domains, contributing significantly to the repulsion and shape. VSEPR Theory Terms and Definitions Bonding Pairs (AX) Electron pairs that are involved in the bonding. Lone Pairs (E) – aka non-bonding pairs or unshared pairs Electrons that are not involved in the bonding. They tend to occupy a larger domain. Electron Domains (ED) Total number of pairs found in the molecule that contribute to its shape. VSEPR – Molecular Shape Multiple covalent bonds Bond Angle: around the same atom • Angle formed by any determine the shape two terminal (outside) Negative e- pairs (same atoms and a central charge) repel each other atom • Caused by the repulsion Repulsions push the pairs as far apart as possible of shared electron pairs. Hybridization What’s a hybrid? • Combining two of the same -
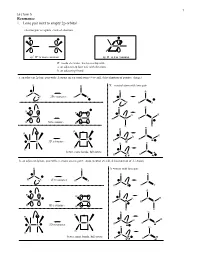
Resonance 1. Lone Pair Next to Empty 2P Orbital
1 Lecture 5 Resonance 1. Lone pair next to empty 2p orbital electron pair acceptors - lack of electrons C C CC sp2 R+ is more common sp R+ is less common R+ needs electrons, has to overlap with a. an adjacent 2p lone pair with electrons b. an adjacent pi bond a. an adjacent 2p lone pair with electrons on a neutral atom (+ overall, delocalization of positive charge) R R X = neutral atom with lone pair R R C C R X 2D resonance R X C C R X R X R R R R C R C R CX CX R N R N R 3D resonance R R R R R R R C R C R CX R O CX 3D resonance R O R R R R C better, more bonds, full octets C R F R F b. an adjacent 2p lone pair with electrons on a negative atom (neutral overall, delocalization of electrons) R R X =anion with lone pair R R C C R X 2D resonance R X C C R X R X R R R R C R C R CX CX R C R C R 3D resonance R R R R R R R C R C R CX R N CX 3D resonance R N R R R R C C better, more bonds, full octets R O R O 2 Lecture 5 Problem 1 – All of the following examples demonstrate delocalization of a lone pair of electrons into an empty 2p orbital. Usually in organic chemistry this is a carbocation site, but not always. -

Effect of Lone Pairs on Hybridization
EFFECT OF LONE PAIRS ON HYBRIDIZATION We have discussed the effect of ghost lone pairs on the shape of a molecule in the post about VSEPR theory. The effect lone pairs exert is the same in VBT as it was in VSEPR. In this post we will study a few examples in which central atom possess lone pairs. First, we will take the example of NH3 molecule. When you draw its Lewis dot structure, you will find three bonding pairs of electrons and one lone pair on N. Now write the ground state configuration of N. 7N: 1s2, 2s2, 2p3 N already has 3 unpaired electrons in ground state to make bond with 3 H atoms. You may think why does N need hybridization in this case when it has 3 unpaired electrons of the same orbital p? Because hybridized orbitals are more competent in overlapping which results in a stronger bond. 3 In NH3 molecule, N chooses sp hybridization. This may get you puzzled again, if N has 3 unpaired electrons in p why does it include s orbital which has paired electrons? You know that the atoms follow Hund’s rule for filling electrons in orbitals, similarly they have to consider energy order of orbitals in hybridization also. When an atom undergoes hybridization, it has to pick orbitals in the increasing order of their energies. In NH3, N has to first pick 2s then 2px, 2py and then 2pz. N can’t ignore 2s even if it is filled because 2s has the lowest energy and N has to include all three 2p orbitals because it needs them for bonding with three H atoms. -

How Do We Know That There Are Atoms
Chemistry 11 Fall 2010 Discussion – 12/13/10 MOs and Hybridization 1. a. [:C N:]- Bond order = (8 – 2) / 2 = 3 *2p * 2p C 2p N 2p 2p 2p *2s C 2s N 2s 2s CN- b. Comparison of Lewis and MO models of CN- CN- Consistent: Both have triple bond (B.O. = 3) Inconsistent: - Lewis structure shows lone pairs on each atom rather than all e- being shared (MO) - Lewis structure indicates a negative formal charge on C, whereas MO suggests more electron density on N, which is consistent with EN considerations c. Both molecules exhibit sp mixing, and the MOs should reflect this. In N2, the molecular orbitals would be distributed equally between the two N atoms, whereas in CN-, the bonding orbitals have greater electron density on the more electronegative atom (N). The antibonding orbitals have more electron density on the less electronegative atom (C). - 1 - Chemistry 11 Fall 2010 2. a. Completed structure of guanine (carbons at each intersection are implied): * * * * b. 17 sigma bonds; 4 pi bonds. c. All carbons are sp2 hybridized; trigonal planar; with 120˚ bond angles. d. We predict that the three N’s marked with * are sp3 hybridized, with 109.5˚ bond angles. The remaining N atoms are sp2 hybridized, with 120˚ bond angles. e. i. Benzene: ii. For the structure drawn in (a), the bond angles in the six membered ring would be expected to be 120˚ for each atom except for the N§, which is predicted to have bond angles of 109.5˚. However, it is not possible to form a planar six-membered ring unless ALL the angles are 120˚. -

Localized Electron Model for Bonding What Does Hybridization Mean?
190 Localized electron model for bonding 1. Determine the Lewis Structure for the molecule 2. Determine resonance structures 3. Determine the shape of the molecule 4. Determine the hybridization of the atoms What does hybridization mean? Why hybridize? Look at the shape of many molecules, and compare this to the shape of the orbitals of each of the atoms. Shape of orbitals Orbitals on atoms are s, p, d, and f, and there are certain shapes associated with each orbital. an s orbital is a big sphere the f orbitals look like two four leaf the p orbitals looks like “8” ’s clovers each one points in along an axis three f orbitals look like weird “8”’s with two doughnuts around each four of the d orbitals look like four one leaf clovers one d orbital looks like an “8” with a doughnut around the middle Shape of molecules Remember we determine the shape of molecules by determining the number of sets of electrons around the atom. count double and triple bonds as one set, count single bonds as one set of electrons and count lone pairs as a set 2 sets—each pair of electrons points 180° away from the other 5 sets—each pair points toward the corner of a trigonal bipyramid 3 sets—each pair points toward the corner of a triangle 6 sets—each pair points toward the corners of an octahedron 4 sets—each pair points toward the corner of a tetrahedron 191 To make bonds we need orbitals—the electrons need to go some where. -

Origin of Chalcogen-Bonding Interactions
Edinburgh Research Explorer The Origin of Chalcogen-Bonding Interactions Citation for published version: Pascoe, D, Ling, K & Cockroft, S 2017, 'The Origin of Chalcogen-Bonding Interactions', Journal of the American Chemical Society, vol. 139, no. 42, pp. 15160–15167. https://doi.org/10.1021/jacs.7b08511 Digital Object Identifier (DOI): 10.1021/jacs.7b08511 Link: Link to publication record in Edinburgh Research Explorer Document Version: Peer reviewed version Published In: Journal of the American Chemical Society General rights Copyright for the publications made accessible via the Edinburgh Research Explorer is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The University of Edinburgh has made every reasonable effort to ensure that Edinburgh Research Explorer content complies with UK legislation. If you believe that the public display of this file breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. Download date: 11. Oct. 2021 The Origin of Chalcogen-Bonding Interactions Dominic J. Pascoe†, Kenneth B. Ling‡, and Scott L. Cockroft†* †EaStCHEM School of Chemistry, University of Edinburgh, Joseph Black Building, David Brewster Road, Edinburgh, EH9 3FJ, UK ‡Syngenta, Jealott’s Hill International Research Centre, Bracknell, Berkshire, RG42 6EY, UK ABSTRACT: Favorable molecular interactions between group 16 elements have been implicated in catalysis, biological processes, materials and medicinal chemistry. Such interactions have since become known as chalcogen bonds by analogy to hydrogen and halogen bonds.