(L) DC and Iris Tectorum Maxim Based on Plant Metabolomics, D
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Isolation, Identification and Characterization of Allelochemicals/Natural Products
Isolation, Identification and Characterization of Allelochemicals/Natural Products Isolation, Identification and Characterization of Allelochemicals/Natural Products Editors DIEGO A. SAMPIETRO Instituto de Estudios Vegetales “Dr. A. R. Sampietro” Universidad Nacional de Tucumán, Tucumán Argentina CESAR A. N. CATALAN Instituto de Química Orgánica Universidad Nacional de Tucumán, Tucumán Argentina MARTA A. VATTUONE Instituto de Estudios Vegetales “Dr. A. R. Sampietro” Universidad Nacional de Tucumán, Tucumán Argentina Series Editor S. S. NARWAL Haryana Agricultural University Hisar, India Science Publishers Enfield (NH) Jersey Plymouth Science Publishers www.scipub.net 234 May Street Post Office Box 699 Enfield, New Hampshire 03748 United States of America General enquiries : [email protected] Editorial enquiries : [email protected] Sales enquiries : [email protected] Published by Science Publishers, Enfield, NH, USA An imprint of Edenbridge Ltd., British Channel Islands Printed in India © 2009 reserved ISBN: 978-1-57808-577-4 Library of Congress Cataloging-in-Publication Data Isolation, identification and characterization of allelo- chemicals/natural products/editors, Diego A. Sampietro, Cesar A. N. Catalan, Marta A. Vattuone. p. cm. Includes bibliographical references and index. ISBN 978-1-57808-577-4 (hardcover) 1. Allelochemicals. 2. Natural products. I. Sampietro, Diego A. II. Catalan, Cesar A. N. III. Vattuone, Marta A. QK898.A43I86 2009 571.9’2--dc22 2008048397 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying or otherwise, without the prior permission of the publisher, in writing. The exception to this is when a reasonable part of the text is quoted for purpose of book review, abstracting etc. -

Relation Structure/Activité De Tanins Bioactifs Contre Les Nématodes
En vue de l'obtention du DOCTORAT DE L'UNIVERSITÉ DE TOULOUSE Délivré par : Institut National Polytechnique de Toulouse (INP Toulouse) Discipline ou spécialité : Pathologie, Toxicologie, Génétique et Nutrition Présentée et soutenue par : Mme JESSICA QUIJADA PINANGO le jeudi 17 décembre 2015 Titre : RELATION STRUCTURE/ACTIVITE DE TANINS BIOACTIFS CONTRE LES NEMATODES GASTROINTESTINAUX (HAEMONCHUS CONTORTUS) PARASITES DES PETITS RUMINANTS Ecole doctorale : Sciences Ecologiques, Vétérinaires, Agronomiques et Bioingénieries (SEVAB) Unité de recherche : Interactions Hôtes - Agents Pathogènes (IHAP) Directeur(s) de Thèse : M. HERVÉ HOSTE Rapporteurs : M. ADIBE LUIZ ABDALLA, UNIVERSIDAD DE SAO PAULO Mme HEIDI ENEMARK, NORWEGIAN VETERINARY INSTITUTE Membre(s) du jury : 1 M. FRANÇOIS SCHELCHER, ECOLE NATIONALE VETERINAIRE DE TOULOUSE, Président 2 M. HERVÉ HOSTE, INRA TOULOUSE, Membre 2 Mme CARINE MARIE-MAGDELAINE, INRA PETIT BOURG, Membre 2 M. SMARO SOTIRAKI, HAO-DEMETER, Membre 2 M. VINCENT NIDERKORN, INRA CLERMONT FERRAND, Membre QUIJADA J. 2015 Cette thèse est dédiée à mes parents, Teresa et Héctor, À mon mari, Rafäel, pour son soutien inconditionnel, son amour illimité, sa patience, sa loyauté, son amitié et surtout sa confidence, À ma grand-mère, Marcolina, car m'ait donné le plus grand et précieux cadeau en ma vie : ma foi en Dieu ma forteresse et mon espoir (Isaïas 41:13). À mes adorés sœurs, belle- sœurs et frère : Yurlin, Indira, Iskay, Olga, Zoraida et Jesus. Merci pour l’amour infini que m’ont toujours été donné, celui qu’a été prolongé par l'amour de mes merveilleux neveux. 1 QUIJADA J. 2015 REMERCIEMENTS Je remercie tout d’abord mon Dieu pour me donner le cadeau de la vie, et la forteresse pour vivre chaque jour. -

IN SILICO ANALYSIS of FUNCTIONAL Snps of ALOX12 GENE and IDENTIFICATION of PHARMACOLOGICALLY SIGNIFICANT FLAVONOIDS AS
Tulasidharan Suja Saranya et al. Int. Res. J. Pharm. 2014, 5 (6) INTERNATIONAL RESEARCH JOURNAL OF PHARMACY www.irjponline.com ISSN 2230 – 8407 Research Article IN SILICO ANALYSIS OF FUNCTIONAL SNPs OF ALOX12 GENE AND IDENTIFICATION OF PHARMACOLOGICALLY SIGNIFICANT FLAVONOIDS AS LIPOXYGENASE INHIBITORS Tulasidharan Suja Saranya, K.S. Silvipriya, Manakadan Asha Asokan* Department of Pharmaceutical Chemistry, Amrita School of Pharmacy, Amrita Viswa Vidyapeetham University, AIMS Health Sciences Campus, Kochi, Kerala, India *Corresponding Author Email: [email protected] Article Received on: 20/04/14 Revised on: 08/05/14 Approved for publication: 22/06/14 DOI: 10.7897/2230-8407.0506103 ABSTRACT Cancer is a disease affecting any part of the body and in comparison with normal cells there is an elevated level of lipoxygenase enzyme in different cancer cells. Thus generation of lipoxygenase enzyme inhibitors have suggested being valuable. Individual variation was identified by the functional effects of Single Nucleotide Polymorphisms (SNPs). 696 SNPs were identified from the ALOX12 gene, out of which 73 were in the coding non-synonymous region, from which 8 were found to be damaging. In silico analysis was performed to determine naturally occurring flavonoids such as isoflavones having the basic 3- phenylchromen-4-one skeleton for the pharmacological activity, like Genistein, Diadzein, Irilone, Orobol and Pseudobaptigenin. O-methylated isoflavones such as Biochanin, Calycosin, Formononetin, Glycitein, Irigenin, 5-O-Methylgenistein, Pratensein, Prunetin, ψ-Tectorigenin, Retusin and Tectorigenine were also used for the study. Other natural products like Aesculetin, a coumarin derivative; flavones such as ajoene and baicalein were also used for the comparative study of these natural compounds along with acteoside and nordihydroguaiaretic acid (antioxidants) and active inhibitors like Diethylcarbamazine, Zileuton and Azelastine as standard for the computational analysis. -

Global Journal of Medical Research
Online ISSN : 2249 - 4618 Print ISSN : 0975 - 5888 Study of Cytological Pattern Development of Animal Models Polymorphism with breast cancer Histopathological and Toxicological effects Volume 12 | Issue 1 | Version 1.0 Global Journal of Medical Research Global Journal of Medical Research Volume 12 Issue 1 (Ver. 1.0) Open Association of Research Society © Global Journal of Medical Global Journals Inc. Research . 2012. (A Delaware USA Incorporation with “Good Standing”; Reg. Number: 0423089) Sponsors: Open Association of Research Society All rights reserved. Open Scientific Standards This is a special issue published in version 1.0 Publisher’s Headquarters office of “Global Journal of Medical Research.” By Global Journals Inc. Global Journals Inc., Headquarters Corporate Office, All articles are open access articles distributed Cambridge Office Center, II Canal Park, Floor No. under “Global Journal of Medical Research” 5th, Cambridge (Massachusetts), Pin: MA 02141 Reading License, which permits restricted use. Entire contents are copyright by of “Global United States Journal of Medical Research” unless USA Toll Free: +001-888-839-7392 otherwise noted on specific articles. USA Toll Free Fax: +001-888-839-7392 No part of this publication may be reproduced Offset Typesetting or transmitted in any form or by any means, electronic or mechanical, including photocopy, recording, or any information Open Association of Research Society , Marsh Road, storage and retrieval system, without written permission. Rainham, Essex, London RM13 8EU United Kingdom. The opinions and statements made in this book are those of the authors concerned. Ultraculture has not verified and neither confirms nor denies any of the foregoing and Packaging & Continental Dispatching no warranty or fitness is implied. -

Dr. Duke's Phytochemical and Ethnobotanical Databases List of Chemicals for Chronic Venous Insufficiency/CVI
Dr. Duke's Phytochemical and Ethnobotanical Databases List of Chemicals for Chronic Venous Insufficiency/CVI Chemical Activity Count (+)-AROMOLINE 1 (+)-CATECHIN 5 (+)-GALLOCATECHIN 1 (+)-HERNANDEZINE 1 (+)-PRAERUPTORUM-A 1 (+)-SYRINGARESINOL 1 (+)-SYRINGARESINOL-DI-O-BETA-D-GLUCOSIDE 1 (-)-ACETOXYCOLLININ 1 (-)-APOGLAZIOVINE 1 (-)-BISPARTHENOLIDINE 1 (-)-BORNYL-CAFFEATE 1 (-)-BORNYL-FERULATE 1 (-)-BORNYL-P-COUMARATE 1 (-)-CANADINE 1 (-)-EPICATECHIN 4 (-)-EPICATECHIN-3-O-GALLATE 1 (-)-EPIGALLOCATECHIN 1 (-)-EPIGALLOCATECHIN-3-O-GALLATE 2 (-)-EPIGALLOCATECHIN-GALLATE 3 (-)-HYDROXYJASMONIC-ACID 1 (-)-N-(1'-DEOXY-1'-D-FRUCTOPYRANOSYL)-S-ALLYL-L-CYSTEINE-SULFOXIDE 1 (1'S)-1'-ACETOXYCHAVICOL-ACETATE 1 (2R)-(12Z,15Z)-2-HYDROXY-4-OXOHENEICOSA-12,15-DIEN-1-YL-ACETATE 1 (7R,10R)-CAROTA-1,4-DIENALDEHYDE 1 (E)-4-(3',4'-DIMETHOXYPHENYL)-BUT-3-EN-OL 1 1,2,6-TRI-O-GALLOYL-BETA-D-GLUCOSE 1 1,7-BIS(3,4-DIHYDROXYPHENYL)HEPTA-4E,6E-DIEN-3-ONE 1 Chemical Activity Count 1,7-BIS(4-HYDROXY-3-METHOXYPHENYL)-1,6-HEPTADIEN-3,5-DIONE 1 1,8-CINEOLE 1 1-(METHYLSULFINYL)-PROPYL-METHYL-DISULFIDE 1 1-ETHYL-BETA-CARBOLINE 1 1-O-(2,3,4-TRIHYDROXY-3-METHYL)-BUTYL-6-O-FERULOYL-BETA-D-GLUCOPYRANOSIDE 1 10-ACETOXY-8-HYDROXY-9-ISOBUTYLOXY-6-METHOXYTHYMOL 1 10-GINGEROL 1 12-(4'-METHOXYPHENYL)-DAURICINE 1 12-METHOXYDIHYDROCOSTULONIDE 1 13',II8-BIAPIGENIN 1 13-HYDROXYLUPANINE 1 14-ACETOXYCEDROL 1 14-O-ACETYL-ACOVENIDOSE-C 1 16-HYDROXY-4,4,10,13-TETRAMETHYL-17-(4-METHYL-PENTYL)-HEXADECAHYDRO- 1 CYCLOPENTA[A]PHENANTHREN-3-ONE 2,3,7-TRIHYDROXY-5-(3,4-DIHYDROXY-E-STYRYL)-6,7,8,9-TETRAHYDRO-5H- -

Phylogenetic Insights on the Isoflavone Profile Variations In
Food Research International 76 (2015) 51–57 Contents lists available at ScienceDirect Food Research International journal homepage: www.elsevier.com/locate/foodres Phylogenetic insights on the isoflavone profile variations in Fabaceae spp.: Assessment through PCA and LDA Tatiana Visnevschi-Necrasov a,b, João C.M. Barreira b,c,⁎,SaraC.Cunhab, Graça Pereira d, Eugénia Nunes a, M. Beatriz P.P. Oliveira b a CIBIO-ICETA, Faculdade de Ciências, Universidade do Porto, R. Padre Armando Quintas 4485-661 Vairão, Portugal b REQUIMTE, Departamento de Ciências Químicas, Faculdade de Farmácia, Universidade do Porto, Rua Jorge Viterbo Ferreira, No. 228, 4050-313, Porto,Portugal c CIMO-ESA, Instituto Politécnico de Bragança, Campus de Santa Apolónia, Apartado 1172, 5301-855 Bragança, Portugal d INRB/IP — INIA — Instituto Nacional de Recursos Biológicos, Caia E São Pedro Estrada Gil Vaz, 7350-228 Elvas, Portugal article info abstract Article history: Legumes (Fabaceae) are important crops, known as sources of food, feed for livestock and raw materials for in- Received 30 September 2014 dustry. Their ability to capture atmospheric nitrogen during symbiotic processes with soil bacteria reduces the Received in revised form 15 November 2014 need for expensive chemical fertilizers, improving soil and water quality. Several Fabaceae species are acknowl- Accepted 20 November 2014 edged for the high levels of secondary metabolites. Isoflavones are among the most well-known examples of Available online 28 November 2014 these compounds, being recognized for their several types of biological activity. Herein, isoflavone profiles were characterized in nine species of four Fabaceae genera (Biserrula, Lotus, Ornithopus and Scorpiurus). Plants Chemical compounds studied in this article: fl Daidzin (PubChem CID: 107971) were harvested in the late ower physiological stage to prevent biased results due to naturally occurring varia- Genistin (PubChem CID: 5281377) tions along the vegetative cycle. -

Download Download
Volume 3, Issue1, January 2012 Available Online at www.ijppronline.in International Journal Of Pharma Professional’s Research Review Article DALBERGIA SISSOO: AN OVERVIEW ISSN NO:0976-6723 Shivani saini*, Dr. Sunil sharma Guru Jambheshwar University of Science and Technology, Hisar, Haryana, India, 125001 Abstract The present review is, therefore, an effort to give a detailed survey of the literature on its pharamacognosy, phytochemistry, traditional uses and pharmacological studies of the plant Dalbergia sissoo. Dalbergia sissoo is an important timber species around the world. Besides this, it has been utilized as medicines for thousands of years and now there is a growing demand for plant based medicines, health products, pharmaceuticals and cosmetics. Dalbergia sissoo is a widely growing plant which is used traditionally as anti-inflammatory, antipyretic, analgesic, anti-oxidant, anti-diabetic and antimicrobial agent. Several phytoconstituents have been isolated and identified from different parts of the plant belonging tothe category of alkaloids, glycosides, flavanols, tannins, saponins, sterols and terpenoids. A review of plant description, phytochemical constituents present and their pharmacological activities are given in the present article. Keywords: - Dalbergia sissoo, phytochemical constituents, pharmacological activities. Introduction medicine.[7] To be accepted as viable alternative to Medicinal plants have been the part and parcel of modern medicine, the same vigorous method of human society to combat diseases since the dawn of scientific and clinical validation must be applied to human civilization. The earliest description of prove the safety and effectiveness of a therapeutic curative properties of medicinal plants were product.[ 8-9] described in the Rigveda (2500-1800 BC), Charak The genus, Dalbergia, consists of 300 species and Samhita and Sushruta Samhita. -

Ep 3138585 A1
(19) TZZ¥_¥_T (11) EP 3 138 585 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 08.03.2017 Bulletin 2017/10 A61L 27/20 (2006.01) A61L 27/54 (2006.01) A61L 27/52 (2006.01) (21) Application number: 16191450.2 (22) Date of filing: 13.01.2011 (84) Designated Contracting States: (72) Inventors: AL AT BE BG CH CY CZ DE DK EE ES FI FR GB • Gousse, Cecile GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO 74230 Dingy Saint Clair (FR) PL PT RO RS SE SI SK SM TR • Lebreton, Pierre Designated Extension States: 74000 Annecy (FR) BA ME •Prost,Nicloas 69440 Mornant (FR) (30) Priority: 13.01.2010 US 687048 26.02.2010 US 714377 (74) Representative: Hoffmann Eitle 30.11.2010 US 956542 Patent- und Rechtsanwälte PartmbB Arabellastraße 30 (62) Document number(s) of the earlier application(s) in 81925 München (DE) accordance with Art. 76 EPC: 15178823.9 / 2 959 923 Remarks: 11709184.3 / 2 523 701 This application was filed on 29-09-2016 as a divisional application to the application mentioned (71) Applicant: Allergan Industrie, SAS under INID code 62. 74370 Pringy (FR) (54) STABLE HYDROGEL COMPOSITIONS INCLUDING ADDITIVES (57) The present specification generally relates to hydrogel compositions and methods of treating a soft tissue condition using such hydrogel compositions. EP 3 138 585 A1 Printed by Jouve, 75001 PARIS (FR) EP 3 138 585 A1 Description CROSS REFERENCE 5 [0001] This patent application is a continuation-in-part of U.S. -

Bioactive Fractions and Compounds from Dalbergia
(19) TZZ ZZ_T (11) EP 2 705 047 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C07H 17/07 (2006.01) A61K 31/7048 (2006.01) 05.08.2015 Bulletin 2015/32 C07D 311/36 (2006.01) A61K 31/352 (2006.01) A61P 19/10 (2006.01) (21) Application number: 12729239.9 (86) International application number: (22) Date of filing: 25.04.2012 PCT/IN2012/000301 (87) International publication number: WO 2012/147102 (01.11.2012 Gazette 2012/44) (54) BIOACTIVE FRACTIONS AND COMPOUNDS FROM DALBERGIA SISSOO FOR THE PREVENTION OR TREATMENT OF OSTEO-HEALTH RELATED DISORDERS BIOAKTIVE FRAKTIONEN UND VERBINDUNGEN AUS DALBERGIA SISSOO ZUR VORBEUGUNG ODER BEHANDLUNG KNOCHENZUSTANDSBEDINGTER ERKRANKUNGEN FRACTIONS ET COMPOSÉS BIOACTIFS ISSUS DE DALBERGIA SISSOO POUR PRÉVENIR OU TRAITER LES TROUBLES D’ORIGINE OSTÉOPATHIQUE (84) Designated Contracting States: • WAHAJUDDIN AL AT BE BG CH CY CZ DE DK EE ES FI FR GB Lucknow 226001 (IN) GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO • JAIN, Girish, Kumar PL PT RO RS SE SI SK SM TR Lucknow 226001 (IN) • CHATTOPADHYAY, Naibedya (30) Priority: 25.04.2011 IN DE12062011 Lucknow 226001 (IN) (43) Date of publication of application: (74) Representative: Jakobsson, Jeanette Helene 12.03.2014 Bulletin 2014/11 Awapatent AB P.O. Box 5117 (73) Proprietor: Council of Scientific & Industrial 200 71 Malmö (SE) Research New Delhi 110 001 (IN) (56) References cited: WO-A2-00/62765 WO-A2-2007/042010 (72) Inventors: FR-A1- 2 483 228 • MAURYA, Rakesh Lucknow 226001 (IN) • PREETY DIXIT ET AL: "Constituents of Dalbergia • DIXIT, Preety sissoo Roxb. -

PDF-Document
Supplementary Materials 1 Metabolomics profiling analysis of red clover Table S1. Compound identification of red clover in positive and negative ion modes. MS1 No RT Ion Ion Measur Predicte Ste Le Flow Err MS2 MS3 Identification . min Form Formula ed d m af er ppm m/z m/z [M − 195.050 1 1.43 C6H11O7 195.0499 2.82 Gluconic acid ++ ++ +++ H]- 5 179.03506(C9H7O4); Benzoylcitronensa [M − 295.045 295.0448 ++ 2 4.46 C13H11O8 0.67 133.01451(C4H5O5); [295-179]135.04524(C8H7O2); ure ++ +++ H]- 0 4 + 115.00400(C4H3O4); [M + 355.102 ++ 3 5.42 C16H19O9 355.1024 1.21 chlorogenic acid + +++ H]+ 8 + [433-271]253.04956(C15H9O4); 243.06529(C14H11O4); [M + 433.111 Genistein- ++ 4 6.60 C21H21O10 433.1129 −3.54 271.06067(C15H11O5); 215.07042(C13H11O3); +++ +++ H]+ 4 glucoside + 153.01839(C7H5O4); 149.02335(C8H5O3); [M + 417.117 5 6.71 C21H21O9 417.1180 −2.34 239.07021(C15H11O3) daidzin + ++ +++ H]+ 0 [M + 447.127 calycosin-7-O-β-D- ++ 6 7.20 C22H23O10 447.1286 −1.91 269.08072(C16H13O4) +++ +++ H]+ 7 glucoside + 1 [533-285]270.05258(C15H10O5); Calycosin-7-O-β- [M + 533.127 253.04982(C15H9O4); ++ 7 8.03 C25H25O13 533.1290 −3.54 285.07642(C16H13O5); D-glucoside 4′′-O- +++ +++ H]+ 1 225.05478(C14H9O3); + malonate 137.02333(C7H5O3); [M + 433.112 ++ 8 8.95 C21H21O10 433.1129 −2.11 255.06516(C15H11O4) genistin ++ +++ H]+ 0 + [519-271]253.04958(C15H9O4,); Genistein- 243.06526(C14H11O4); [M + 519.111 433.11377(C21H21O10); glucoside ++ 9 9.31 C24H23O13 519.1133 −3.25 215.07045(C13H11O3); - +++ H]+ 6 271.06073(C15H11O5); malonate + 153.01833(C7H5O4); 149.02333(C8H5O3); -

Isolation and Identification of Chemical Constituents from Zhideke Granules by Ultra-Performance Liquid Chromatography Coupled with Mass Spectrometry
Hindawi Journal of Analytical Methods in Chemistry Volume 2020, Article ID 8889607, 16 pages https://doi.org/10.1155/2020/8889607 Research Article Isolation and Identification of Chemical Constituents from Zhideke Granules by Ultra-Performance Liquid Chromatography Coupled with Mass Spectrometry Guangqiang Huang ,1 Jie Liang ,1,2,3 Xiaosi Chen,1 Jing Lin,1 Jinyu Wei,1 Dongfang Huang,1 Yushan Zhou,1 Zhengyi Sun,4 and Lichun Zhao 1,3 1College of Pharmacy, Guangxi University of Chinese Medicine, Nanning 530200, China 2Guangxi Key Laboratory of Zhuang and Yao Ethnic Medicine, Nanning 530200, China 3Guangxi Zhuang Yao Medicine Center of Engineering and Technology, Nanning 530200, China 4Ruikang Hospital Affiliated to Guangxi University of Chinese Medicine, Nanning 530011, China Correspondence should be addressed to Jie Liang; [email protected] and Lichun Zhao; [email protected] Received 25 August 2020; Accepted 8 December 2020; Published 29 December 2020 Academic Editor: Luca Campone Copyright © 2020 Guangqiang Huang et al. ,is is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Chemical constituents from Zhideke granules were rapidly isolated and identified by ultra-performance liquid chromatography (UPLC) coupled with hybrid quadrupole-orbitrap mass spectrometry (MS) in positive and negative ion modes using both full scan and two-stage threshold-triggered mass modes. ,e secondary fragment ion information of the target compound was selected and compared with the compound reported in databases and related literatures to further confirm the possible compounds. A total of 47 chemical constituents were identified from the ethyl acetate extract of Zhideke granules, including 21 flavonoids and glycosides, 9 organic acids, 4 volatile components, 3 nitrogen-containing compounds, and 10 other compounds according to the frag- mentation patterns, relevant literature, and MS data. -
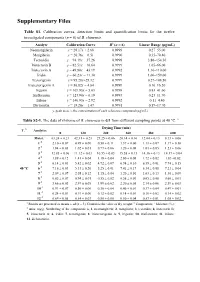
Supplementary Files
Supplementary Files Table S1. Calibration curves, detection limits and quantification limits for the twelve investigated components (n = 6) of B. chinensis. Analyte Calibration Curve R2 (n = 6) Linear Range (μg/mL) Neomangiferin y = 28.17x + 2.68 0.9999 0.27–55.00 Mangiferin y = 20.76x − 6.51 0.9990 0.35–70.40 Tectoridin y = 94.19x − 37.26 0.9998 3.86–154.50 Iristectorin B y = 82.51x − 10.64 0.9999 1.65–66.00 Iristectorin A y = 49.80x − 43.19 0.9992 1.10–110.00 Iridin y = 60.23x − 11.30 0.9999 1.06–159.00 Tectorigenin y = 95.28x+25.32 0.9999 0.27–108.50 Iristectorigenin A y = 56.82x − 4.84 0.9999 0.16–16.20 Irigenin y = 109.93x + 5.03 0.9998 0.83–41.60 Irisflorentin y = 123.90x − 0.19 0.9993 0.23–11.70 Irilone y = 146.93x − 2.92 0.9992 0.11–4.40 Dictomitin y = 18.20x − 1.47 0.9991 0.59–17.70 y, peak area; x, the concentration of each reference compound (μg/mL). Table S2-1. The data of rhizome of B. chinensis in G1 from different sampling points at 40 °C. a Drying Time (min) T. b Analytes 0 120 240 360 480 600 Moist. c 63.24 ± 0.21 42.31 ± 0.23 27.25 ± 0.06 20.14 ± 0.10 12.04 ± 0.13 8.13 ± 0.06 1 d 2.10 ± 0.07 0.95 ± 0.00 0.50 ± 0.11 1.37 ± 0.00 1.11 ± 0.07 1.17 ± 0.10 2 d 1.84 ± 0.03 1.02 ± 0.01 0.77 ± 0.06 1.20 ± 0.08 1.03 ± 0.05 1.23 ± 0.06 3 d 12.85 ± 0.06 11.12 ± 0.03 10.55 ± 0.03 15.58 ± 0.13 14.36 ± 0.13 14.17 ± 0.04 4 d 1.89 ± 0.12 1.41 ± 0.04 1.19 ± 0.04 2.00 ± 0.08 1.72 ± 0.02 1.83 ±0.02 5 d 6.14 ± 0.03 5.42 ± 0.02 4.72 ± 0.07 6.94 ± 0.10 6.59 ± 0.01 7.74 ± 0.15 40 °C 6 d 7.16 ± 0.03 5.33 ± 0.26 5.25 ± 0.01 7.41 ± 0.17 6.34 ±