GLOBAL OFFERING (Incorporated in the Cayman Islands with Limited Liability)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Wuxi Biologics (Cayman) Inc. 藥明生物技術有限公司*
THIS CIRCULAR IS IMPORTANT AND REQUIRES YOUR IMMEDIATE ATTENTION If you are in any doubt as to any aspect of this circular or as to the action to be taken, you should consult a stockbroker or other registered dealer in securities, a bank manager, solicitor, professional accountant or other professional advisor. If you have sold or transferred all your shares in WuXi Biologics (Cayman) Inc., you should at once hand this circular, together with the enclosed form of proxy, to the purchaser or transferee or to the bank, stockbroker or other agent through whom the sale or transfer was effected for transmission to the purchaser or transferee. Hong Kong Exchanges and Clearing Limited and The Stock Exchange of Hong Kong Limited take no responsibility for the contents of this circular, make no representation as to its accuracy or completeness and expressly disclaim any liability whatsoever for any loss howsoever arising from or in reliance upon the whole or any part of the contents of this circular. WUXI BIOLOGICS (CAYMAN) INC. * 藥明生物技術有限公司 (Incorporated in the Cayman Islands with limited liability) (Stock Code: 2269) (1) PROPOSED RE-ELECTION OF RETIRING DIRECTORS (2) PROPOSED ELECTION OF NEW DIRECTOR (3) CONNECTED TRANSACTION INVOLVING GRANT OF RESTRICTED SHARES TO CONNECTED PERSONS PURSUANT TO SPECIFIC MANDATE (4) PROPOSED GRANT OF GENERAL MANDATES TO ISSUE SHARES AND TO REPURCHASE SHARES AND (5) NOTICE OF ANNUAL GENERAL MEETING Independent Financial Advisor to the Independent Shareholders The notice convening the Annual General Meeting of WuXi Biologics (Cayman) Inc. to be held at the meeting room of Sheraton Shanghai Waigaoqiao Hotel, 28 Jilong Road, Pilot Free Trade Zone, Shanghai, China on Wednesday, June 16, 2021 at 3:00 p.m. -

Hs-10352 Tablets”
Hong Kong Exchanges and Clearing Limited and The Stock Exchange of Hong Kong Limited take no responsibility for the contents of this announcement, make no representation as to its accuracy or completeness and expressly disclaim any liability whatsoever for any loss howsoever arising from or in reliance upon the whole or any part of the contents of this announcement. Hansoh Pharmaceutical Group Company Limited 翰森製藥集團有限公司 (Incorporated in the Cayman Islands with limited liability) (Stock Code: 3692) VOLUNTARY ANNOUNCEMENT CLINICAL TRIAL NOTICE OF “HS-10352 TABLETS” The board of directors (the “Board”) of Hansoh Pharmaceutical Group Company Limited (the “Company” and together with its subsidiaries, the “Group”) is pleased to announce that “HS-10352 tablets”, a Category 1 innovative drug developed by Jiangsu Hansoh Pharmaceutical Group Co., Ltd. (江蘇豪森藥業集團有限公司) and Shanghai Hansen Technology Co., Ltd. (上 海翰森生物醫藥科技有限公司), subsidiaries of the Company, was granted a clinical trial notice issued by the National Medical Products Administration of the People’s Republic of China on May 18, 2020, and is intended to be used for the treatment of patients with a phosphatidylinositol-4, 5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA+) mutation in hormone receptor-positive (HR+) and human epidermal growth factor receptor 2 negative (HER2-) advanced breast cancer. The final indication will be determined after the completion of clinical research. By Order of the Board Hansoh Pharmaceutical Group Company Limited Zhong Huijuan Chairlady Hong Kong, May 18, 2020 As at the date of this announcement, the Board comprises Ms. Zhong Huijuan as chairlady and executive director, Mr. Lyu Aifeng and Miss Sun Yuan as executive directors, Ms. -

The History of Gyalthang Under Chinese Rule: Memory, Identity, and Contested Control in a Tibetan Region of Northwest Yunnan
THE HISTORY OF GYALTHANG UNDER CHINESE RULE: MEMORY, IDENTITY, AND CONTESTED CONTROL IN A TIBETAN REGION OF NORTHWEST YUNNAN Dá!a Pejchar Mortensen A dissertation submitted to the faculty at the University of North Carolina at Chapel Hill in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Department of History. Chapel Hill 2016 Approved by: Michael Tsin Michelle T. King Ralph A. Litzinger W. Miles Fletcher Donald M. Reid © 2016 Dá!a Pejchar Mortensen ALL RIGHTS RESERVED ii! ! ABSTRACT Dá!a Pejchar Mortensen: The History of Gyalthang Under Chinese Rule: Memory, Identity, and Contested Control in a Tibetan Region of Northwest Yunnan (Under the direction of Michael Tsin) This dissertation analyzes how the Chinese Communist Party attempted to politically, economically, and culturally integrate Gyalthang (Zhongdian/Shangri-la), a predominately ethnically Tibetan county in Yunnan Province, into the People’s Republic of China. Drawing from county and prefectural gazetteers, unpublished Party histories of the area, and interviews conducted with Gyalthang residents, this study argues that Tibetans participated in Communist Party campaigns in Gyalthang in the 1950s and 1960s for a variety of ideological, social, and personal reasons. The ways that Tibetans responded to revolutionary activists’ calls for political action shed light on the difficult decisions they made under particularly complex and coercive conditions. Political calculations, revolutionary ideology, youthful enthusiasm, fear, and mob mentality all played roles in motivating Tibetan participants in Mao-era campaigns. The diversity of these Tibetan experiences and the extent of local involvement in state-sponsored attacks on religious leaders and institutions in Gyalthang during the Cultural Revolution have been largely left out of the historiographical record. -

UC GAIA Chen Schaberg CS5.5-Text.Indd
Idle Talk New PersPectives oN chiNese culture aNd society A series sponsored by the American Council of Learned Societies and made possible through a grant from the Chiang Ching-kuo Foundation for International Scholarly Exchange 1. Joan Judge and Hu Ying, eds., Beyond Exemplar Tales: Women’s Biography in Chinese History 2. David A. Palmer and Xun Liu, eds., Daoism in the Twentieth Century: Between Eternity and Modernity 3. Joshua A. Fogel, ed., The Role of Japan in Modern Chinese Art 4. Thomas S. Mullaney, James Leibold, Stéphane Gros, and Eric Vanden Bussche, eds., Critical Han Studies: The History, Representation, and Identity of China’s Majority 5. Jack W. Chen and David Schaberg, eds., Idle Talk: Gossip and Anecdote in Traditional China Idle Talk Gossip and Anecdote in Traditional China edited by Jack w. cheN aNd david schaberg Global, Area, and International Archive University of California Press berkeley los Angeles loNdoN The Global, Area, and International Archive (GAIA) is an initiative of the Institute of International Studies, University of California, Berkeley, in partnership with the University of California Press, the California Digital Library, and international research programs across the University of California system. University of California Press, one of the most distinguished university presses in the United States, enriches lives around the world by advancing scholarship in the humanities, social sciences, and natural sciences. Its activities are supported by the UC Press Foundation and by philanthropic contributions from individuals and institutions. For more information, visit www.ucpress.edu. University of California Press Berkeley and Los Angeles, California University of California Press, Ltd. -

Chronology of Chinese History
AppendixA 1257 Appendix A Chronology of Chinese History Xla Dynasty c. 2205 - c. 1766 B. C. Shang Dynasty c. 1766 - c. 1122 B. C. Zhou Dynasty c. 1122 - 249 B. C. Western Zhou c. 1122 - 771 B.C. Eastern Zhou 770 - 249 B. C. Spring Autumn and period 770 - 481 B.C. Warring States period 403 - 221 B.C. Qin Dynasty 221 - 207 B. C. Han Dynasty 202 B. C. - A. D. 220 Western Han 202 B.C. -AD. 9 Xin Dynasty A. D. 9-23 Eastern Han AD. 25 - 220 Three Kingdoms 220 - 280 Wei 220 - 265 Shu 221-265 Wu 222 - 280 Jin Dynasty 265 - 420 Western Jin 265 - 317 Eastern Jin 317 - 420 Southern and Northern Dynasties 420 - 589 Sui Dynasty 590 - 618 Tang Dynasty 618 - 906 Five Dynasties 907 - 960 Later Liang 907 - 923 Later Tang 923 - 936 Later Jin 936 - 947 Later Han 947 - 950 Later Zhou 951-960 Song Dynasty 960-1279 Northern Song 960-1126 Southern Song 1127-1279 Liao 970-1125 Western Xia 990-1227 Jin 1115-1234 Yuan Dynasty 1260-1368 Ming Dynasty 1368-1644 Cling Dynasty 1644-1911 Republic 1912-1949 People's Republic 1949- 1258 Appendix B Map of China C ot C x VV 00 aý 3 ýý, cý ýý=ý<<ý IAJ wcsNYý..®c ýC9 0 I Jz ýS txS yQ XZL ý'Tl '--} -E 0 JVvýc ý= ' S .. NrYäs Zw3!v )along R ?yJ L ` (Yana- 'ý. ý. wzX: 0. ý, {d Q Z lýý'? ý3-ýý`. e::. ý z 4: `ý" ý i kws ". 'a$`: ýltiCi, Ys'ýlt.^laS-' tý.. -

Hansoh Pharmaceutical Group Company Limited 翰森製藥集團有限公司 (Incorporated in the Cayman Islands with Limited Liability) (Stock Code: 3692)
Hong Kong Exchanges and Clearing Limited and The Stock Exchange of Hong Kong Limited take no responsibility for the contents of this announcement, make no representation as to its accuracy or completeness and expressly disclaim any liability whatsoever for any loss howsoever arising from or in reliance upon the whole or any part of the contents of this announcement. Hansoh Pharmaceutical Group Company Limited 翰森製藥集團有限公司 (Incorporated in the Cayman Islands with limited liability) (Stock Code: 3692) INSIDE INFORMATION LICENSE AGREEMENT WITH EQRX This announcement is made by Hansoh Pharmaceutical Group Company Limited (the “Company”, together with its subsidiaries, the “Group”) pursuant to Rule 13.09(2)(a) of the Rules Governing the Listing of Securities on The Stock Exchange of Hong Kong Limited (the “Listing Rules”) and the inside information provision (as defined in the Listing Rules) under Part XIVA of the Securities and Futures Ordinance (Cap. 571). The board (the “Board”) of directors (the “Directors”) of the Company is pleased to announce that on July 23, 2020 (before trading hours), Hansoh (Shanghai) Healthtech Co., Ltd. (翰森(上海)健 康科技有限公司) and Jiangsu Hansoh Pharmaceutical Group Company Ltd. (江蘇豪森藥業集團 有限公司) (collectively, the “Licensors”), each a wholly-owned subsidiary of the Group, entered into a strategic collaboration and license agreement (the “License Agreement”) with EQRx, INC. (“EQRx”), pursuant to which the Licensors grant, among others, an exclusive license to permit EQRx to research, develop, manufacture and commercialize Almonertinib and any product containing or comprising of Almonertinib in the field of treatment of cancer, cancer-related and immune-inflammatory diseases in humans outside of the People’s Republic of China (“PRC”). -

World Adds 346 Billionaires to Take Total to Record
WORLD ADDS 346 BILLIONAIRES TO TAKE TOTAL TO RECORD 2,816 BILLIONAIRES CHINA AND USA LEAD WITH 799 AND 626 BILLIONAIRES, MAKING UP OVER HALF OF ’KNOWN’ BILLIONAIRES IN WORLD. INDIA, GERMANY AND UK FOLLOW WITH 100+ EACH. 479 NEW FACES AND 130 DROP OFFS CHINA 182 NEW FACES, DESPITE TRADE WAR, THREE TIMES THAT OF USA WITH 59 NEW FACES JEFF BEZOS, 56, OF AMAZON, RICHEST MAN IN WORLD FOR THIRD YEAR RUNNING, WITH US$140BN, DOWN US$7BN. EX-WIFE MACKENZIE MAKES LIST FOR FIRST TIME WITH US$44BN AT 22ND PLACE. PARIS-BASED BERNARD ARNAULT UP US$21BN TO 2ND PLACE WITH US$107BN, ON BACK OF SURGE IN LVMH SHARE PRICE. RECORD FOUR INDIVIDUALS CROSS US$100BN CUT-OFF. BILL GATES AND WARREN BUFFETT IN THIRD AND FOURTH PLACES WITH US$106BN AND US$102BN. TOP 10 HAS US$1TN. ELON MUSK HAS A STORMING YEAR, UP 10 PLACES TO 20TH WITH US$46BN, ON BACK OF TESLA SHARE PRICE DOUBLING, OVERTAKING CHINA NUMBER ONE JACK MA OF ALIBABA ON US$45BN DESPITE US-CHINA TRADE WAR, REN ZHENGFEI OF HUAWEI UP 7% TO US$3BN, SAME AS DONALD TRUMP AT 900TH IN THE WORLD. COSMETICS QUEEN KYLIE JENNER, 22, YOUNGEST SELF-MADE BILLIONAIRE WITH US$1.1BN ADAM NEUMANN, 40, OF WEWORK BIGGEST LOSER, WITH WEALTH DOWN 80% TO US$1.3BN BEIJING IS WORLD‘S BILLIONAIRE CAPITAL FOR 5TH YEAR RUNNING WITH 110 BILLIONAIRES LIVING THERE, 12 AHEAD OF NEW YORK. SHANGHAI OVERTAKE HONG KONG. CORONOVIRUS DRIVES UP STOCKS OF CHINESE ONLINE EDUCATION, ONLINE GAMES AND PHARMACEUTICAL COMPANIES DOING VACCINES. -

恒投證券 Hengtou Securities
Hong Kong Exchanges and Clearing Limited and The Stock Exchange of Hong Kong Limited take no responsibility for the contents of this announcement, make no representation as to its accuracy or completeness and expressly disclaim any liability whatsoever for any loss howsoever arising from or in reliance upon the whole or any part of the contents of this announcement. 恒投證券 HENGTOU SECURITIES (a joint stock company incorporated in the People’s Republic of China with limited liability under the Chinese corporate name “恒泰證券股份有限公司” and carrying on business in Hong Kong as “恒投證券” (in Chinese) and “HENGTOU SECURITIES” (in English)) (the “Company”) (Stock code: 01476) ANNUAL RESULTS ANNOUNCEMENT FOR THE YEAR ENDED 31 DECEMBER 2016 The board of directors (the “Board”) of the Company hereby announces the audited annual results of the Company and its subsidiaries for the year ended 31 December 2016. This announcement, containing the full text of the 2016 annual report of the Company, complies with the relevant requirements of the Rules Governing the Listing of Securities on The Stock Exchange of Hong Kong Limited in relation to information to accompany preliminary announcement of annual results and has been reviewed by the audit committee of the Company. The Board resolved that no profit distribution will be made for the year ended 31 December 2016. PUBLICATION OF ANNUAL RESULTS ANNOUNCEMENT AND ANNUAL REPORT This annual results announcement will be published on the websites of The Stock Exchange of Hong Kong Limited (www.hkexnews.hk) and the Company (www.cnht.com.cn). The 2016 annual report of the Company will be dispatched to the shareholders of the Company and published on the websites of The Stock Exchange of Hong Kong Limited and the Company in due course but no later than the end of April 2017. -
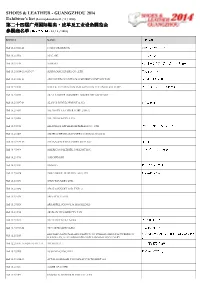
SLG14 Exhibitors List 23 May 2014 (Website).Xlsx
SHOES & LEATHER - GUANGZHOU 2014 Exhibitor's list (Last updated on 23 / 5 / 2014) 第二十四届广州州国际国际鞋鞋类类类、、、皮革及工皮革及工业设备展展览会览会 参参展商名展商名单 (最后更新日期: 23 / 5 / 2014) BOOTH NAME 公司名称称称 Hall 11.2/0521-22 169 INFORMATION 169 资讯《鞋业&皮革信息》 Hall 10.2/0703 A.P.C. SRL 阿皮奇有限公司 Hall 10.2/0719 A.SHOES 東莞市華瑞世界鞋業總部基地有限公司 Hall 11.2/0114-16, 0135-37 ADINA MACHINERY CO., LTD. 艾特拿有限公司 Hall 11.2/0426-28 ADVANCED OCTOPUS MACHINERY COMPANY LTD 洋逸机械发展有限公司 Hall 11.2/0833 AITECK AUTOMATION INTEGRATION TECHNOLOGY CORP. 鸿宇自动化设备股份有限公司 Hall 10.2/0319 AKA LEATHER INDUSTRY AND TRADE COMPANY Hall 10.2/0537-38 ALAN\'S DEVELOPMENT & CO. 锦华发展公司 Hall 10.2/0313 ALL RIGHT LEATHER CORP. (ARLC) Hall 11.2/0205 ALL TRENDS PTE. LTD. Hall 11.2/0733 ALLFINELY APPLIED MATERIALS CO., LTD. 欧桦应用材料股份有限公司 Hall 11.2/0307 ALLIED CHEMICALS INTERNATIONAL CO.,LTD. Hall 11.2/0435-36 AMANN & SOEHNE GMBH & CO. KG 亚曼集团 Hall 10.2/0428 AMERICAN BILTRITE FAR EAST INC. 美國普得利上海代表處 Hall 10.2/0733 AMICHEM SRL Hall 12.2/0421 ANWOO 广州安予贸易有限公司 Hall 11.2/0324 AOKAI SHOE MATERIAL CO.,LTD 奥凯鞋材有限公司 Hall 10.2/0331 APEX TANNERY LTD. Hall 10.2/0332 APEX TANNERY LTD. UNIT-2 Hall 12.2/0335 ARES ITALIA S.R.L. Hall 11.2/0528 ARS ARPEL SCHOOL & MAGAZINES Hall 10.2/0715 ARTISAN LEATHER PVT LTD Hall 11.2/0622 ASIA FOOTWERA NEWS 《亚洲鞋业》杂志 Hall 10.2/0203-04 ASIA STAR CHINA LTD. 立辉中国有限公司 ASSOMAC-NATIONAL ASSOCIATION OF ITALIAN MANUFACTURERS OF 阿索玛科- 意大利制鞋,制革, 制皮具机械协 Hall 12.2/0215 FOOTWEAR, LEATHERGOODS AND TANNING MACHINERY 会 Hall 12.2/0111-15, 0230-34, 0311-14 ATOM S.P.A. -

Sun Piaoyang
LE PORTRAITLes DUmeilleurs MOIS fonds actions des marchés émergents (au 31/03/2018) retenus GemEquitysur la base des meilleursInvestir profils sur les risque/rendements actions émergentes à long terme. Les noms de sociétés sont donnés à titre d’exemples, ni leur présence dans le portefeuille, ni leur performance ne sont garanties. Le présent document est publié à titre d’information uniquement. Il ne constitue ni un document contractuel, ni une incitation à l’investissement. Il est destiné à des investisseurs professionnels et ne peut être diffusé à un tiers sans accord préalable de Gemway Assets SAS. GemEquity est majoritairement investi en actions et présente un risque de perte en capital. Pour plus d’informations sur GemEquity, vous pouvez contacter votre interlocuteur habituel et vous référer au DICI et au prospectus sur www.gemway.com. «Ces tycoons qui contribuent au succès des pays émergents» Sun Piaoyang un chercheur émérite et innovant À 60 ans, le Président de Jiangsu Hengrui Medicine et sa femme sont classés 15ème Jiangsu Hengrui de la China Rich List 2018. Leur fortune s’élève à 9,9 Md$ (source : Forbes 2019). Medicine, Sun Piaoyang est né dans la ville de Lianyungang en Chine. Elève brillant, il obtient tout d’abord un bachelor à la China Pharmaceutical University en 1982 et termine par un doctorat en chimie organique Quelques chiffres à la Nanjing University. L’éducation fait partie intégrante de la success story de Sun Piaoyang. Peu de temps avant la fin de son doctorat, Sun commence à travailler dans l’usine pharmaceutique de Lianyungang Lianyungagn, une entreprise d’état, en tant que technicien. -

Comparison of the Grain Quality and Starch Physicochemical Properties
agriculture Article Comparison of the Grain Quality and Starch Physicochemical Properties between Japonica Rice Cultivars with Different Contents of Amylose, as Affected by Nitrogen Fertilization Yajie Hu , Shumin Cong and Hongcheng Zhang * Jiangsu Co-Innovation Center for Modern Production Technology of Grain Crops, Jiangsu Key Laboratory of Crop Genetics and Physiology, Yangzhou University, Yangzhou 225009, China; [email protected] (Y.H.); [email protected] (S.C.) * Correspondence: [email protected] Abstract: In order to determine the effects of nitrogen fertilizer on the grain quality and starch physic- ochemical properties of japonica rice cultivars with different contents of amylose, normal amylose content (NAC) and low amylose content (LAC) cultivars were grown in a field, with or without nitrogen fertilizer (WN). The relationships between the amylose content, starch physicochemical properties and eating quality were also examined. Compared with WN, nitrogen fertilizer (NF) significantly increased the grain yield but markedly decreased the grain weight. In addition, the processing quality tended to improve, but the appearance quality and eating quality deteriorated under NF application. The grain yield was similar between NAC and LAC cultivars. However, the grain quality and starch physicochemical properties were significantly different between NAC and LAC cultivars. The palatability of the cooked rice was significantly higher in the LAC than in NAC Citation: Hu, Y.; Cong, S.; Zhang, H. cultivar, which was due to its lower amylose content, protein content, hardness, and retrogradation Comparison of the Grain Quality and enthalpy and degree, and its higher stickiness, peak viscosity, breakdown, relative crystallinity and Starch Physicochemical Properties peak intensity. The amylose content and protein content were significantly negatively correlated with between Japonica Rice Cultivars with Different Contents of Amylose, as the palatability. -

2020 Annual Report 3 Corporate Overview
Hansoh Pharmaceutical Group Company Limited 翰森製藥集團有限公司 (Incorporated in the Cayman Islands with limited liability) Stock Code : 3692 Annual Report 2020 Contents 2 Corporate Overview 5 Corporate Information 7 Financial Highlights 8 Chairlady’s Statement 9 Management Discussion and Analysis 17 Corporate Governance Report 27 Directors’ Report 37 Biographical Details of Directors and Senior Management 43 Independent Auditor’s Report 48 Consolidated Statement of Profit or Loss 49 Consolidated Statement of Comprehensive Income 50 Consolidated Statement of Financial Position 52 Consolidated Statement of Changes in Equity 53 Consolidated Statement of Cash Flows 55 Notes to the Financial Statements Corporate Overview The Company is one of the leading research and development-driven pharmaceutical companies in the People’s Republic of China (“PRC” or “China”), devoting itself to meet the unmet clinical needs of patients and improve the health and well-being of human beings through continuing innovation. The Company has established a leading position in some of the largest and fastest-growing therapeutic areas in the PRC with significant unmet clinical needs, including oncology, anti-infectives, central nervous system (“CNS”) diseases and diabetes. The core driving force of the Company is its focus on innovation. The Company has continuously increased its investments in research and development (“R&D”) over the years, established sound R&D platforms and mastered a number of proprietary technologies. It has successfully launched and developed a series of innovative drugs and first-to-market generic drugs. During the year under review, the Company successfully launched 10 new drugs in total in both PRC and overseas, including self- developed innovative drugs, Ameile (almonertinib mesylate tablets) and Olanzapine Oral Fast Dissolving Films, and 3 first-to-market generic drugs.