Hyaluronic Acid and a Short Peptide Improve the Performance of a PCL Electrospun Fibrous Scaffold Designed for Bone Tissue Engineering Applications
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
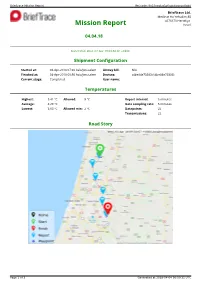
Mission Report Ref.Code: 9N15novkq0w0ggs4ogcwc8g44
Brieftrace Mission Report Ref.code: 9n15novkq0w0ggs4ogcwc8g44 Brieftrace Ltd. Medinat Ha-Yehudim 85 4676670 Herzeliya Mission Report Israel 04.04.18 clalit Status: Success Generated: Wed, 04 Apr 18 09:50:31 +0300 Shipment Configuration Started at: 04-Apr-2018 07:30 Asia/Jerusalem Airway bill: N/A Finished at: 04-Apr-2018 09:50 Asia/Jerusalem Devices: c4be84e73383 (c4be84e73383) Current stage: Completed User name: Shai Temperatures Highest: 5.41 °C Allowed: 8 °C Report interval: 5 minutes Average: 4.29 °C Data sampling rate: 5 minutes Lowest: 3.63 °C Allowed min: 2 °C Datapoints: 22 Transmissions: 22 Road Story Page 1 of 3 Generated at 2018-04-04 06:50:31 UTC Brieftrace Mission Report Ref.code: 9n15novkq0w0ggs4ogcwc8g44 Report Data 13 ° 12 ° 11 ° 10 ° 9 ° Allowed high: 8° C 8 ° C 7 ° ° High e r 6 ° Max: 5.41° C u t a 5 ° r Low e p 4 ° Min: 3.63° C m e 3 ° T Allowed low: 2° C 2 ° 1 ° 0 ° -1 ° -2 ° -3 ° 07:30 07:45 08:00 08:15 08:30 08:45 09:00 09:15 09:30 09:45 Date and time. All times are in Asia/Jerusalem timezone. brieftrace.com Tracker Time Date Temp°CAlert? RH % Location Control Traceability c4be84e73383 07:30 04-04-2018 4.26 79.65 Ha-Matekhet St 17, Kadima Zoran, Israel 636158 dfb902 c4be84e73383 07:36 04-04-2018 4.24 79.75 Ha-Matekhet St 17, Kadima Zoran, Israel 636161 b23bc8 c4be84e73383 07:43 04-04-2018 4.21 79.75 Ha-Matekhet St 17, Kadima Zoran, Israel 636167 c1c01c c4be84e73383 07:49 04-04-2018 4.18 79.85 Ha-Matekhet St 17, Kadima Zoran, Israel 636176 4a3752 c4be84e73383 07:56 04-04-2018 4.21 79.85 Ha-Matekhet St 17, Kadima -

Curriculum Vitae
CURRICULUM VITAE Name: Shabtay Shayke Bilu Date & place of birth: December 7, 1961, Kefar Hanagid, Israel. Citizenship: Israeli. Marital status: Married +3. Affiliation: SCE - Shamoon College of Engineering, 84 Jabotinsky Street, Ashdod, 7724500. Tel: +972-8-6475699 E-mail: [email protected] Residence address: 138 Hela St. Farm 15, Moshav Kefar Hanagid, 7687500, Israel. Tel: +972-8-9421291 Mobile: +972-54-6543598 Telefax: +972-8-9437540. Military service: 1980-1981 I.A.F. Fighter Airplane Ground Mechanical Technician. 1981-1982 I.A.F. Missile & Ammunition Battalion Officer (Lieutenant), 1982-1984 I.A.F. Missile & Ammunition Brigade Officer (Captain), 1984-1986 I.A.F. Head of Missile & Ammunition Section Officer (Major), 1986-1988 Deputy Head of the Human Resources Department at the Air Force Headquarters (Major), 1989-2006 I.D.F. Reserve Officer (Major), 2006- Reserve duty exempt. 1. Academic education 2010-2015 Ph.D. in Management in Education. Anglia Ruskin University, Chelmsford, U.K. Dissertation title: “Stakeholders’ perceptions of appropriate management methods: The case of A. youth-village undergoing change “ Advisers: Dr Simon Pratt-Adams, Dr Jaki Lilly and Prof Gary Peckham. 1998-1999 M.Ed. in Management in Education. Derby University, Derby, U.K. Dissertation title: “The perception of the employees involved in a technological and educational organization concerning the appropriate management method for the organization“. Advisers: Dr Ohela Avinir, Dr Hanna Bar Yishay. 1997-1998 B.A. in Psychology & Education. Burlington College, Vermont, USA. Dissertation title: “Review and comparison of therapeutic approaches: Psychoanalytic, Behavioural, Cognitive and Biomedical relation to the treatment of phobias". Advisers: Prof Gabriel Kovac, Dr Merav Hermesh and Mr Yair Vana. -

Israel National Report for Habitat III National Israel Report
Israel National Report for Habitat III National Report Israel National | 1 Table of content: Israel National Report for Habitat III Forward 5-6 I. Urban Demographic Issues and Challenges for a New Urban Agenda 7-15 1. Managing rapid urbanization 7 2. Managing rural-urban linkages 8 3. Addressing urban youth needs 9 4. Responding to the needs of the aged 11 5. Integrating gender in urban development 12 6. Challenges Experienced and Lessons Learned 13 II. Land and Urban Planning: Issues and Challenges for a New Urban Agenda 16-22 7. Ensuring sustainable urban planning and design 16 8. Improving urban land management, including addressing urban sprawl 17 9. Enhancing urban and peri-urban food production 18 10. Addressing urban mobility challenges 19 11. Improving technical capacity to plan and manage cities 20 Contributors to this report 12. Challenges Experienced and Lessons Learned 21 • National Focal Point: Nethanel Lapidot, senior division of strategic planing and policy, Ministry III. Environment and Urbanization: Issues and Challenges for a New Urban of Construction and Housing Agenda 23-29 13. Climate status and policy 23 • National Coordinator: Hofit Wienreb Diamant, senior division of strategic planing and policy, Ministry of Construction and Housing 14. Disaster risk reduction 24 • Editor: Dr. Orli Ronen, Porter School for the Environment, Tel Aviv University 15. Minimizing Transportation Congestion 25 • Content Team: Ayelet Kraus, Ira Diamadi, Danya Vaknin, Yael Zilberstein, Ziv Rotem, Adva 16. Air Pollution 27 Livne, Noam Frank, Sagit Porat, Michal Shamay 17. Challenges Experienced and Lessons Learned 28 • Reviewers: Dr. Yodan Rofe, Ben Gurion University; Dr. -
Ziv Medical Center Greets the New Director Farewell to Prof
Ziv Newsletter January 2015 Best wishes for a healthy and peaceful 2015 to our friends and benefactors in Israel and around the world. Ziv Medical Center Greets the New Director Farewell to Prof. Oscar Embon Dr. Salman Zarka graduated After 21 years as Director of Ziv from the Faculty of Medicine, Medical Center, Prof. Oscar Embon Technion – Israel Institute of leaves Ziv Medical Center satisfied Technology, Haifa in 1988. He that much has been accomplished received a Master's degree in during his tenure. Prof. Embon, Public Health (MPH) from the faced the challenges of managing Hebrew University of Jerusalem, a hospital in northern Israel, and an additional Master's degree characterized by the complex needs of the region's unique in Political Science from the mosaic of residents as well as those resulting from the University of Haifa. hospital's proximity to both the Lebanese and Syrian borders. Prior to his position at Ziv, Dr. Zarka served as a Colonel in the "Among the projects I am most thrilled about are the I.D.F. for 25 years in a variety of positions, the last of which was construction of the Child Health Center and the Radiotherapy commander of the Military Health Services Department. Previous Unit. The Child Health Center is going to be a magnificent state- to this position, Dr. Zarka was the Head of the Medical Corps of of-the-art partially reinforced building, which will house all of the Northern Command and the Commander of the Military Field the services essential for top quality treatment of children, as Hospital for the Syrian casualties in the Golan Heights. -

I. CARMI, Y. NOTER, and R. SCHLESINGER the Rehovot
[RADIOCARBON, VOL. 13, No. 2, 1971, P. 412-419] REI-I®VOT RADIOCARBON MEASUREMENTS I I. CARMI, Y. NOTER, and R. SCHLESINGER Department of Isotope Research, Weizmann Institute of Science, Rehovot, Israel The Rehovot Radiocarbon Laboratory was established in 1968, as an extension of a low-level tritium laboratory, which has been in opera- tion many years. Intended to be a supporting facility in geohydrological studies, the laboratory now offers general services in carbon dating. For measurements, we use proportional gas counting of ethane, at 2100 torr. The sample counter is a modified RCL counter, of 1.1 L volume; it is operated at 5600 volts. The counter is surrounded, respec- tively, by a Johnston GRC-13 anticoincidence guard counter, 2 cm old lead, 10 cm boron loaded paraffin, and 25 cm pre-2nd-world-war steel. Samples are counted in four channels, in anticoincidence with the guard counter. The four channels count disintegrations between the following energies: channel l: 1 to 18 keV, channel 2: 18 to 59 keV, channel 3: 59 to 155 keV, and channel 4: above 155 keV. C14 is counted in the two middle channels; channel 1 is rejected against possible tritium con- tamination, and channel 4 is used to detect Radon contamination. The working point is determined by coincidence counting of charged cosmic particles: the ratio of count rates in the two sample channels is adjusted to 1. The acquisition and processing of counting data is done automati- cally by an on-line computer (Carmi and Ashkenazi, 1970). Background samples are prepared from alabaster or from anthracite. -

HTLV-1 Associated Adult T-Cell Leukemia/Lymphoma in Israel
M. Shtalrid et al. ization of laboratory tests. Treatment course was compli- HTLV-1 Associated Adult T-cell cated by recurrent episodes of chemotherapy-induced Leukemia/Lymphoma in Israel: report of two neutropenia and infection, including Corynebacterium patients of Romanian origin sepsis, which were successfully treated with colony stim- ulating factor (G-CSF) and antibiotics. Haematologica 2005; 90:(4)e36-e38 In July 2001, three months following her initial presen- Human T-lymphotropic virus type 1 (HTLV-1) was the tation, the patient developed fever, recurrent hypercal- first human oncovirus isolated by Gallo et al in 19801 and cemia 16 mg/dl and generalized maculopapular rash. A established as an etiological agent for adult T-cell skin biopsy revealed infiltration of the dermis by atypical leukemia/ lymphoma (ATL).2 Although more than 15 mil- lymphocytes with the same profile (CD2, CD3, CD4). lion individuals are infected by HTLV-1 through the Sixty percent of the cells were also positive for Ki-67, a world, the spread of the virus is highly endemic. The marker of high proliferative index. The patient did not HTLV-1 infection is prevailing in southwestern Japan, respond to chemotherapy with cytarabine, cyclophos- inter-tropical Africa, Central and South America.3 In phamide, high-dose methotrexate and fludarabine and Kyushu district, Japan, the seroprevalence reaches >30% died. in the adult population. In the US, Europe and the Middle Case 2. A 56-year-old female was born in Bucharest, Romania and immigrated to Israel East the HTLV-1 infection is very rare, and cases of ATL 16 years ago. -

Staring Back at the Sun: Video Art from Israel, 1970-2012 an Exhibition and Public Program Touring Internationally, 2016-2017
Staring Back at the Sun: Video Art from Israel, 1970-2012 An Exhibition and Public Program Touring Internationally, 2016-2017 Roee Rosen, still from Confessions Coming Soon, 2007, video. 8:40 minutes. Video, possibly more than any other form of communication, has shaped the world in radical ways over the past half century. It has also changed contemporary art on a global scale. Its dual “life” as an agent of mass communication and an artistic medium is especially intertwined in Israel, where artists have been using video artistically in response to its use in mass media and to the harsh reality video mediates on a daily basis. The country’s relatively sudden exposure to commercial television in the 1990s coincided with the Palestinian uprising, or Intifada, and major shifts in internal politics. Artists responded to this in what can now be considered a “renaissance” of video art, with roots traced back to the ’70s. An examination of these pieces, many that have rarely been presented outside Israel, as well as recent, iconic works from the past two decades offers valuable lessons on how art and culture are shaped by larger forces. Staring Back at the Sun: Video Art from Israel, 1970-2012 traces the development of contemporary video practice in Israel and highlights work by artists who take an incisive, critical perspective towards the cultural and political landscape in Israel and beyond. Showcasing 35 works, this program includes documentation of early performances, films and videos, many of which have never been presented outside of Israel until now. Informed by the international 1 history of video art, the program surveys the development of the medium in Israel and explores how artists have employed technology and material to examine the unavoidable and messy overlap of art and politics. -

West Nile Virus (WNV) Activity in Humans and Mosquitos
West Nile virus (WNV) activity in humans and mosquitos Summary of 2018 Following is a summary of human cases, animals, birds and mosquitos positive for WNV by location for 2018. In 2018, 147 human cases of West Nile Fever (WNF) were reported in Israel, of which 62 were suspected*. As expected, most of the morbidity occurred during the summer and autumn months, with over 50 patients in August alone. The level of morbidity was higher than the 2011-2017 average. Most of the patients this year resided in the Coastal Plain area. The distribution of patients by district was: North (19), Haifa (38), Central (54), Tel- Aviv (27), Jerusalem (1) and South (8). A significant increase was noted in the number of infected animals as compared to the last few years. אגף לאפידמיולוגיה Division of Epidemiology משרד הבריאות Ministry of Health ת.ד.1176 ירושלים P.O.B 1176 Jerusalem [email protected] [email protected] טל: 02-5080522 פקס: Tel: 972-2-5080522 Fax: 972-2-5655950 02-5655950 In 2018, WNV infected mosquitos were found in 42 locations in the country. In the middle of June in the Valley of Springs at the Northern District and in Yehuda Plains; in July, in the Jordan Rift Valley in addition to the Valley of Springs and in Rehovot area, in August, in the Jerusalem area and in the Coastal Plain; in September, in the Galilee, the Hula Valley, in the Jerusalem area and in the Coastal Plain; in October, in the Arabah Valley and in the Samaria area; and in November, in the Arabah Valley. -

Return of Organization Exempt from Income
Return of Organization Exempt From Income Tax Form 990 Under section 501 (c), 527, or 4947( a)(1) of the Internal Revenue Code (except black lung benefit trust or private foundation) 2005 Department of the Treasury Internal Revenue Service ► The o rganization may have to use a copy of this return to satisfy state re porting requirements. A For the 2005 calendar year , or tax year be and B Check If C Name of organization D Employer Identification number applicable Please use IRS change ta Qachange RICA IS RAEL CULTURAL FOUNDATION 13-1664048 E; a11gne ^ci See Number and street (or P 0. box if mail is not delivered to street address) Room/suite E Telephone number 0jretum specific 1 EAST 42ND STREET 1400 212-557-1600 Instruo retum uons City or town , state or country, and ZIP + 4 F nocounwro memos 0 Cash [X ,camel ded On° EW YORK , NY 10017 (sped ► [l^PP°ca"on pending • Section 501 (Il)c 3 organizations and 4947(a)(1) nonexempt charitable trusts H and I are not applicable to section 527 organizations. must attach a completed Schedule A ( Form 990 or 990-EZ). H(a) Is this a group return for affiliates ? Yes OX No G Website : : / /AICF . WEBNET . ORG/ H(b) If 'Yes ,* enter number of affiliates' N/A J Organization type (deckonIyone) ► [ 501(c) ( 3 ) I (insert no ) ] 4947(a)(1) or L] 527 H(c) Are all affiliates included ? N/A Yes E__1 No Is(ITthis , attach a list) K Check here Q the organization' s gross receipts are normally not The 110- if more than $25 ,000 . -

Jerusalem: Facts and Trends 2009 / 2010
Jerusalem Institute for Israel Studies Founded by the Charles H. Revson Foundation Jerusalem: Facts and Trends 2009 / 2010 Maya Choshen, Michal Korach 2010 Jerusalem Institute for Israel Studies Publication No. 402 Jerusalem: Facts and Trends 2009/2010 Maya Choshen, Michal Korach This publication was published with the assistance of the Charles H. Revson Foundation, New York The authors alone are responsible for the contents of the publication Translation from Hebrew: Sagir International Translation, Ltd. © 2010, Jerusalem Institute for Israel Studies The Hay Elyachar House 20 Radak St., 92186 Jerusalem [email protected] http://www.jiis.org Table of Contents About the Authors ............................................................................................. 7 Preface ................................................................................................................ 8 Area .................................................................................................................... 9 Population ......................................................................................................... 9 Population size ........................................................................................... 9 Geographical distribution of the population .............................................11 Population growth .................................................................................... 12 Sources of population growth .................................................................. 12 Birth -

Israel's Natural Gas Resources: Economic and Strategic Significance
Volume 13 | No. 1 | July 2010 Israel’s Natural Gas Resources: Economic and Strategic Significance |Shmuel Even US-Israel Relations: Approaching a Turning Point? | Zaki Shalom Israel and the US: That Bad? | Oded Eran A Military Attack on Iran? Considerations for Israeli Decision Making | Ron Tira Turning Point 4: The National Civilian Front Exercise | Meir Elran Syria’s Return to Lebanon: The Challenge of the Lebanese State and the Role of Hizbollah | Daniel Sobelman Beyond the Nuclear and Terror Threats: The Conventional Military Balance in the Gulf | Yoel Guzansky 1 ÈÓ‡φÔÂÁËÈ·†È¯˜ÁÓφÔÂÎÓ‰ THE INSTITUTE FOR NATIONAL SECURITY STUDIES INCORPORATING THE JAFFEE CENTER FOR STRATEGIC STUDIES AT TEL AVIV UNIVERSITY ·È·‡≠Ï˙†˙ËÈÒ¯·È‡ ÈÓ‡φÔÂÁËÈ·†È¯˜ÁÓφÔÂÎÓ‰ THE INSTITUTE FOR NATIONAL SECURITY STUDIES INCORPORATING THE JAFFEE CENTER FOR STRATEGIC STUDIES AT TEL AVIV UNIVERSITY ·È·‡≠Ï˙†˙ËÈÒ¯·È‡· Strategic ASSESSMENT Volume 13 | No. 1 | July 2010 COntents Abstracts | 3 Israel’s Natural Gas Resources: Economic and Strategic Significance | 7 Shmuel Even US-Israel Relations: Approaching a Turning Point? | 21 Zaki Shalom Israel and the US: That Bad? | 37 Oded Eran A Military Attack on Iran? Considerations for Israeli Decision Making | 45 Ron Tira Turning Point 4: The National Civilian Front Exercise | 61 Meir Elran Syria’s Return to Lebanon: The Challenge of the Lebanese State and the Role of Hizbollah | 71 Daniel Sobelman Beyond the Nuclear and Terror Threats: The Conventional Military Balance in the Gulf | 85 Yoel Guzansky The purpose of Strategic Assessment is to stimulate and Strategic enrich the public debate on issues that are, or should be, ASSESSMENT on Israel’s national security agenda. -

Nadav Assor Curriculum Vitae
Nadav Assor / Curriculum Vitae [email protected] | www.nadassor.net Selected Exhibitions & Screenings 2014 Ophan, Koffler Arts Center, Toronto, Canada (coming up) Solo show, Juliem Gallery, Tel Aviv (coming up) Afterglow, Transmediale 2014, Berlin, Germany 2013 Ruins of the Map, Gallery 66, Connecticut College, USA Director's Lounge 2013, Contemporary Art Ruhr, Germany Future Perfect Gallery, Singapore Oodaaq Festival, Rennes, France MIA Screening Series, The Armory Center for the Arts, Pasadena, CA, USA Urban Research at Directors Lounge, Berlin , Germany Berlin Director's Lounge Main Program, Berlin, Germany 2012 Co-Recreating Spaces, CentralTrak Gallery, Dallas, TX Simultan Festival, Romania 2011 Young Artist Award Winners, Petah Tikva Museum of Contemporary Art, Israel The Hairy Blob, Hyde Park Art Center, Chicago Sonic Views, Minshar Gallery, Tel Aviv, Israel Conflux, Pearl Conrad Gallery, Ohio State University, USA Transmediale 2012 Festival, Berlin Bangkok Experimental Film Festival, Bangkok, Thailand 2011 Videotheque, Art Toronto International Art Fair, Canada Seret, Solo show at Julie M. gallery, Tel aviv, Israel The Simulationists, International symposium for Mixed Reality performance, Chicago Countdown, the Diaghilev, Tel Aviv, Israel 2010 Effervescent Condition, School of the Art institute of Chicago, Chicago The Power of Copying, installation at Xuzhou museum, China MFA thesis show, Sullivan Galleries, School of the Art institute of Chicago, Chicago 2009 Architecture Inside/Out, Julie M. Gallery, Tel Aviv, Israel New Work, Sullivan Galleries, Chicago Factory, large scale installation at Bat Yam Museum for Contemporary Art, Israel Art of Emergency , Artneuland, Berlin, Germany 2007 Secret Art, Leumi Bank Headquarters, Tel Aviv, Israel Sleep, Russano Gallery, Rishon LeZion, Israel RockArt, Jerusalem Music Center, Jerusalem, Israel Uri 83, Tel Aviv, Israel Dani's House, Tel Aviv, Israel Vidance International Video Dance Festival, Tel Aviv, Israel Camo, Solo show at Julie M.