ORGANIC CHEMISTRY [MH5; Chapter 22, Tutorial Notes]
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cinnamoyl Esters of Lesquerella and Castor Oil: Novel Sunscreen Active Ingredients David L
Cinnamoyl Esters of Lesquerella and Castor Oil: Novel Sunscreen Active Ingredients David L. Compton*, Joseph A. Laszlo, and Terry A. Isbell New Crops and Processing Technology Research Unit, USDA, ARS, NCAUR, Peoria, Illinois 61604 ABSTRACT: Lesquerella and castor oils were esterified with querolin (2). Therefore, LO in essence contains two moles of cinnamic acid (CA) and 4-methoxycinnamic acid (MCA). Esteri- hydroxy functionality per mole of triglyceride (TG). fication of the hydroxy oils reached 85% completion with CA The hydroxy functionality of the FA of the TG can be ex- and 50% conversion with MCA. The hydroxy oils were esteri- ploited by esterification to form estolides. The majority of re- fied at 200°C under a nitrogen atmosphere within a sealed sys- search has focused on the estolides of hydroxy FA and TG tem. Unreacted CA and MCA were removed from the reaction formed with oleic acid. The esterification of hydroxy TG and mixtures by sublimation at 100°C under vacuum. The resultant free lesquerolic acid with oleic acid using a cobalt catalyst (4) methoxycinnamic oils possessed a broader, more blue-shifted or a lipase catalyst (5) has been reported. Also, the acid-cat- UV absorbance, 250 to 345 nm with a λmax of 305 nm, com- pared with the cinnamic oils, which absorbed from 260 to 315 alyzed formation of estolides from CO and LO with oleic acid has been patented (6). Recently, a detailed study of the effect nm, λmax of 270 nm. The methoxycinnamic oils provide better UV-B absorption and thus are better candidates to be used as of oleic acid concentration and temperature on catalyst-free sunscreen active ingredients. -

Molecular Geometry Is the General Shape of a Molecule, As Determined by the Relative Positions of the Atomic Nuclei
Lecture Presentation Chapter 9 Molecular Geometries and Bonding Theories © 2012 Pearson Education, Inc. Chapter Goal • Lewis structures do not show shape and size of molecules. • Develop a relationship between two dimensional Lewis structure and three dimensional molecular shapes • Develop a sense of shapes and how those shapes are governed in large measure by the kind of bonds exist between the atoms making up the molecule © 2012 Pearson Education, Inc. Molecular geometry is the general shape of a molecule, as determined by the relative positions of the atomic nuclei. Copyright © Cengage Learning. All rights reserved. 10 | 3 The valence-shell electron-pair repulsion (VSEPR) model predicts the shapes of molecules and ions by assuming that the valence-shell electron pairs are arranged about each atom so that electron pairs are kept as far away from one another as possible, thereby minimizing electron pair repulsions. The diagram on the next slide illustrates this. Copyright © Cengage Learning. All rights reserved. 10 | 4 Two electron pairs are 180° apart (a linear arrangement). Three electron pairs are 120° apart in one plane (a trigonal planar arrangement). Four electron pairs are 109.5° apart in three dimensions (a tetrahedral arrangment). Copyright © Cengage Learning. All rights reserved. 10 | 5 Five electron pairs are arranged with three pairs in a plane 120° apart and two pairs at 90°to the plane and 180° to each other (a trigonal bipyramidal arrangement). Six electron pairs are 90° apart (an octahedral arrangement). This is illustrated on the next slide. Copyright © Cengage Learning. All rights reserved. 10 | 6 Copyright © Cengage Learning. All rights reserved. -

VSEPR Theory
VSEPR Theory The valence-shell electron-pair repulsion (VSEPR) model is often used in chemistry to predict the three dimensional arrangement, or the geometry, of molecules. This model predicts the shape of a molecule by taking into account the repulsion between electron pairs. This handout will discuss how to use the VSEPR model to predict electron and molecular geometry. Here are some definitions for terms that will be used throughout this handout: Electron Domain – The region in which electrons are most likely to be found (bonding and nonbonding). A lone pair, single, double, or triple bond represents one region of an electron domain. H2O has four domains: 2 single bonds and 2 nonbonding lone pairs. Electron Domain may also be referred to as the steric number. Nonbonding Pairs Bonding Pairs Electron domain geometry - The arrangement of electron domains surrounding the central atom of a molecule or ion. Molecular geometry - The arrangement of the atoms in a molecule (The nonbonding domains are not included in the description). Bond angles (BA) - The angle between two adjacent bonds in the same atom. The bond angles are affected by all electron domains, but they only describe the angle between bonding electrons. Lewis structure - A 2-dimensional drawing that shows the bonding of a molecule’s atoms as well as lone pairs of electrons that may exist in the molecule. Provided by VSEPR Theory The Academic Center for Excellence 1 April 2019 Octet Rule – Atoms will gain, lose, or share electrons to have a full outer shell consisting of 8 electrons. When drawing Lewis structures or molecules, each atom should have an octet. -

Synthesis of Polycyclic Natural Products Jianmin Shi Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1990 Synthesis of polycyclic natural products Jianmin Shi Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Organic Chemistry Commons Recommended Citation Shi, Jianmin, "Synthesis of polycyclic natural products " (1990). Retrospective Theses and Dissertations. 9891. https://lib.dr.iastate.edu/rtd/9891 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. JLÎMI MICROFILMED 1991 INFORMATION TO USERS The most advanced technology has been used to photograph and reproduce this manuscript from the microfihn master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand corner and continuing from left to right in equal sections with small overlaps. -

Sample Exercise 9.1 Using the VSEPR Model
Sample Exercise 9.1 Using the VSEPR Model – Use the VSEPR model to predict the molecular geometry of (a) O3, (b) SnCl3 . Solution Analyze: We are given the molecular formulas of a molecule and a polyatomic ion, both conforming to the general formula ABn and both having a central atom from the p block of the periodic table. Plan: To predict the molecular geometries of these species, we first draw their Lewis structures and then count the number of electron domains around the central atom. The number of electron domains gives the electron-domain geometry. We then obtain the molecular geometry from the arrangement of the domains that are due to bonds. (a) We can draw two resonance structures for O3: Because of resonance, the bonds between the central O atom and the outer O atoms are of equal length. In both resonance structures the central O atom is bonded to the two outer O atoms and has one nonbonding pair. Thus, there are three electron domains about the central O atoms. (Remember that a double bond counts as a single electron domain.) The arrangement of three electron domains is trigonal planar (Table 9.1). Two of the domains are from bonds, and one is due to a nonbonding pair. So, the molecule has a bent shape with an ideal bond angle of 120° (Table 9.2). Chemistry: The Central Science, Eleventh Edition Copyright ©2009 by Pearson Education, Inc. By Theodore E. Brown, H. Eugene LeMay, Bruce E. Bursten, and Catherine J. Murphy Upper Saddle River, New Jersey 07458 With contributions from Patrick Woodward All rights reserved. -

Unit One Part 2: Naming and Functional Groups
gjr-–- 1 Unit One Part 2: naming and functional groups • To write and interpret IUPAC names for small, simple molecules • Identify some common functional groups found in organic molecules O CH3 H3C N H N O N N O H3C S N O N H3C viagra™ (trade name) sildenafil (trivial name) 5-(2-ethoxy-5-(4-methylpiperazin-1-ylsulfonyl)phenyl)-1-methyl-3-propyl-1H- pyrazolo[4,3-d] pyrimidin-7(6H)-one dr gareth rowlands; [email protected]; science tower a4.12 http://www.massey.ac.nz/~gjrowlan gjr-–- 2 Systematic (IUPAC) naming PREFIX PARENT SUFFIX substituents / minor number of C principal functional functional groups AND multiple bond group index • Comprises of three main parts • Note: multiple bond index is always incorporated in parent section No. Carbons Root No. Carbons Root Multiple-bond 1 meth 6 hex Bond index 2 eth 7 hept C–C an(e) 3 prop 8 oct C=C en(e) 4 but 9 non C≡C yn(e) 5 pent 10 dec gjr-–- 3 Systematic (IUPAC) naming: functional groups Functional group Structure Suffix Prefix General form O –oic acid acid –carboxylic acid carboxy R-COOH R OH O O –oic anhydride anhydride –carboxylic anhydride R-C(O)OC(O)-R R O R O –oyl chloride acyl chloride -carbonyl chloride chlorocarbonyl R-COCl R Cl O –oate ester –carboxylate alkoxycarbonyl R-COOR R OR O –amide amide –carboxamide carbamoyl R-CONH2 R NH2 nitrile R N –nitrile cyano R-C≡N O –al aldehyde –carbaldehyde oxo R-CHO R H O ketone –one oxo R-CO-R R R alcohol R OH –ol hydroxy R-OH amine R NH2 –amine amino R-NH2 O ether R R –ether alkoxy R-O-R alkyl bromide bromo R-Br (alkyl halide) R Br (halo) (R-X) gjr-–- 4 Nomenclature rules 1. -

Polymer Exemption Guidance Manual POLYMER EXEMPTION GUIDANCE MANUAL
United States Office of Pollution EPA 744-B-97-001 Environmental Protection Prevention and Toxics June 1997 Agency (7406) Polymer Exemption Guidance Manual POLYMER EXEMPTION GUIDANCE MANUAL 5/22/97 A technical manual to accompany, but not supersede the "Premanufacture Notification Exemptions; Revisions of Exemptions for Polymers; Final Rule" found at 40 CFR Part 723, (60) FR 16316-16336, published Wednesday, March 29, 1995 Environmental Protection Agency Office of Pollution Prevention and Toxics 401 M St., SW., Washington, DC 20460-0001 Copies of this document are available through the TSCA Assistance Information Service at (202) 554-1404 or by faxing requests to (202) 554-5603. TABLE OF CONTENTS LIST OF EQUATIONS............................ ii LIST OF FIGURES............................. ii LIST OF TABLES ............................. ii 1. INTRODUCTION ............................ 1 2. HISTORY............................... 2 3. DEFINITIONS............................. 3 4. ELIGIBILITY REQUIREMENTS ...................... 4 4.1. MEETING THE DEFINITION OF A POLYMER AT 40 CFR §723.250(b)... 5 4.2. SUBSTANCES EXCLUDED FROM THE EXEMPTION AT 40 CFR §723.250(d) . 7 4.2.1. EXCLUSIONS FOR CATIONIC AND POTENTIALLY CATIONIC POLYMERS ....................... 8 4.2.1.1. CATIONIC POLYMERS NOT EXCLUDED FROM EXEMPTION 8 4.2.2. EXCLUSIONS FOR ELEMENTAL CRITERIA........... 9 4.2.3. EXCLUSIONS FOR DEGRADABLE OR UNSTABLE POLYMERS .... 9 4.2.4. EXCLUSIONS BY REACTANTS................ 9 4.2.5. EXCLUSIONS FOR WATER-ABSORBING POLYMERS........ 10 4.3. CATEGORIES WHICH ARE NO LONGER EXCLUDED FROM EXEMPTION .... 10 4.4. MEETING EXEMPTION CRITERIA AT 40 CFR §723.250(e) ....... 10 4.4.1. THE (e)(1) EXEMPTION CRITERIA............. 10 4.4.1.1. LOW-CONCERN FUNCTIONAL GROUPS AND THE (e)(1) EXEMPTION................. -

Polyphenolic Compounds Extracted and Purified from Buddleja Globosa
molecules Article Polyphenolic Compounds Extracted and Purified from Buddleja Globosa Hope (Buddlejaceae) Leaves Using Natural Deep Eutectic Solvents and Centrifugal Partition Chromatography Jeniffer Torres-Vega 1 , Sergio Gómez-Alonso 2 , José Pérez-Navarro 2 , Julio Alarcón-Enos 3 and Edgar Pastene-Navarrete 1,3,* 1 Laboratorio de Farmacognosia, Departamento de Farmacia, Facultad de Farmacia, Universidad de Concepción, Concepción PC4030000, Chile; [email protected] 2 Regional Institute for Applied Scientific Research, Faculty of Chemical Sciences, University of Castilla-La Mancha, PC13071 Castilla-La Mancha, Spain; [email protected] (S.G.-A.); [email protected] (J.P.-N.) 3 Laboratorio de Síntesis y Biotransformación de Productos Naturales, Universidad del Bío-Bío, Chillán PC3800708, Chile; [email protected] * Correspondence: [email protected]; Tel.: +56-(42)-246-3000 Abstract: Chemical profiling of Buddleja globosa was performed by high-performance liquid chro- matography coupled to electrospray ionization (HPLC-DAD-ESI-IT/MS) and quadrupole time-of-flight high-resolution mass spectrometry (HPLC-ESI-QTOF/MS). The identification of 17 main phenolic com- pounds in B. globosa leaf extracts was achieved. Along with caffeoyl glucoside isomers, caffeoylshikimic Citation: Torres-Vega, J.; acid and several verbascoside derivatives (β-hydroxyverbascoside and β-hydroxyisoverbascoside) were Gómez-Alonso, S.; Pérez-Navarro, J.; Alarcón-Enos, J.; Pastene-Navarrete, identified. Among flavonoid compounds, the presence of 6-hydroxyluteolin-7-O-glucoside, quercetin-3- E. Polyphenolic Compounds O-glucoside, luteolin 7-O-glucoside, apigenin 7-O-glucoside was confirmed. Campneoside I, forsytho- Extracted and Purified from Buddleja side B, lipedoside A and forsythoside A were identified along with verbascoside, isoverbascoside, Globosa Hope (Buddlejaceae) Leaves eukovoside and martynoside. -

Studies on the Chemistry of Paclitaxel
STUDIES ON THE CHEMISTRY OF PACLITAXEL Haiqing Yuan Dissertation submitted to the Faculty of the Virginia Polytechnic Institute and State University in the partial fulfillment of the requirement for the degree of Doctor of Philosophy in Chemistry Dr. David G. I. Kingston, Chair Dr. Michael Calter Dr. Neal Castagnoli, Jr. Dr. Richard Gandour Dr. Larry Taylor August 11, 1998 Blacksburg, Virginia Keywords: Paclitaxel, Taxol®, synthesis, analog, SAR Copyright 1998, Haiqing Yuan STUDIES ON THE CHEMISTRY OF PACLITAXEL HAIQING YUAN (ABSTRACT) Paclitaxel is a natural occurring diterpene alkaloid originally isolated from the bark of Taxus brevifolia. It is now one of the most important chemotherapeutic agents for clinical treatment of ovarian and breast cancers. Recent clinical trials have also shown paclitaxel’s potential for the treatment of non-small-cell lung cancer, head and neck cancer, and other types of cancers. While tremendous chemical research efforts have been made in the past years, which established the fundamental structure-activity relationships of the paclitaxel molecule, and provided analogs for biochemical studies to elucidate the precise mechanism of action and for the development of second-generation agents, many areas remain to be explored. In continuation of our efforts in the structure-activity relationships study of A- norpaclitaxel, five new analogs modified at the C-1 substituent and analogs with expanded B-ring or contracted C-ring have now been prepared. Preliminary biological studies indicated that the volume rather than functionality at the C-1 position plays a role in determining the anticancer activity by controlling the relative position of the tetracyclic ring system, which in turn controls the positions of the most critical functionalities such as the C-2 benzoyl, the C-4 acetate, and the C-13 side chain. -

Electron Ionization
Chapter 6 Chapter 6 Electron Ionization I. Introduction ......................................................................................................317 II. Ionization Process............................................................................................317 III. Strategy for Data Interpretation......................................................................321 1. Assumptions 2. The Ionization Process IV. Types of Fragmentation Pathways.................................................................328 1. Sigma-Bond Cleavage 2. Homolytic or Radical-Site-Driven Cleavage 3. Heterolytic or Charge-Site-Driven Cleavage 4. Rearrangements A. Hydrogen-Shift Rearrangements B. Hydride-Shift Rearrangements V. Representative Fragmentations (Spectra) of Classes of Compounds.......... 344 1. Hydrocarbons A. Saturated Hydrocarbons 1) Straight-Chain Hydrocarbons 2) Branched Hydrocarbons 3) Cyclic Hydrocarbons B. Unsaturated C. Aromatic 2. Alkyl Halides 3. Oxygen-Containing Compounds A. Aliphatic Alcohols B. Aliphatic Ethers C. Aromatic Alcohols D. Cyclic Ethers E. Ketones and Aldehydes F. Aliphatic Acids and Esters G. Aromatic Acids and Esters 4. Nitrogen-Containing Compounds A. Aliphatic Amines B. Aromatic Compounds Containing Atoms of Nitrogen C. Heterocyclic Nitrogen-Containing Compounds D. Nitro Compounds E. Concluding Remarks on the Mass Spectra of Nitrogen-Containing Compounds 5. Multiple Heteroatoms or Heteroatoms and a Double Bond 6. Trimethylsilyl Derivative 7. Determining the Location of Double Bonds VI. Library -
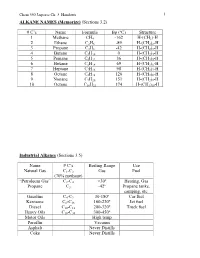
C's Name Formula Bp (ºC) Structure 1 Methane CH4 -162 H-(CH2)
Chem 350 Jasperse Ch. 3 Handouts 1 ALKANE NAMES (Memorize) (Sections 3.2) # C’s Name Formula Bp (ºC) Structure 1 Methane CH4 -162 H-(CH2)-H 2 Ethane C2H6 -89 H-(CH2)2-H 3 Propane C3H8 -42 H-(CH2)3-H 4 Butane C4H10 0 H-(CH2)4-H 5 Pentane C5H12 36 H-(CH2)5-H 6 Hexane C6H14 69 H-(CH2)6-H 7 Heptane C7H16 98 H-(CH2)7-H 8 Octane C8H18 126 H-(CH2)8-H 9 Nonane C9H20 151 H-(CH2)9-H 10 Octane C10H22 174 H-(CH2)10-H Industrial Alkanes (Sections 3.5) Name # C’s Boiling Range Use Natural Gas C1-C3 Gas Fuel (70% methane) “Petroleum Gas” C2-C4 <30º Heating, Gas Propane C3 -42º Propane tanks, camping, etc. Gasoline C4-C9 30-180º Car fuel Kerosene C8-C16 160-230º Jet fuel Diesel C10-C18 200-320º Truck fuel Heavy Oils C16-C30 300-450º Motor Oils High temp Paraffin Vacuum Asphalt Never Distills Coke Never Distills Chem 350 Jasperse Ch. 3 Handouts 2 Nomenclature of Alkanes (Sections 3.3) Systematic IUPAC Rules for Branched and Substituted Alkanes (Section 3.3B) 1. Longest continuous C-chain “core name” 2. Number core chain from an end nearest a substituent 3. Name substituents as “alkyl” groups: 4. Specify the location of substituents using numbers (hyphenate the #’s) • If >2 substituents, list alphabetically • Use di-, tri-, tetra- if the same substituent is repeated. (But ignore these in alphabetizing). Punctuation Notes: • Hyphenate numbers • Do not put a space between substituents and the core name Special Names for Some 3 or 4-carbon Substituents H3C CH3 CH Memorize H3C C H3C CH3 Isopropyl t-butyl or tert-butyl H2 H2 CH3 H3C C H3C C CH H3C CH Others C C C C CH3 H3C C H2 H H H2 2 2 H2 n-propyl n-butyl isobutyl s-butyl (n for "normal") Another Classification System Primary (1º): with one attached carbon Secondary (2º): with two attached carbons Tertiary (3º): with three attached carbons H C C C C C C C C H 1º H 2º C 3º Very Complex Substituents (Not responsible) Substituent: (1-ethyl-2,3-dimethylpentyl) Overall: 9-(1-ethyl-2,3-dimethylpentyl)nonadecane Chem 350 Jasperse Ch. -

Organic Chemistry Tests for Hydroxyl Group
Chemistry Organic Chemistry Tests for Hydroxyl Group General Aim Method Identication of aliphatic alcohols through the chemical Detection of the presence of hydroxyl groups in detection of hydroxyl groups. aliphatic alcohols using special chemical tests. Learning Objectives (ILOs) Dene and determine aliphatic alcohols theoretically through their chemical structure. Classify organic compounds containing hydroxyl groups into aliphatic and aromatic. Compare between alcohols and other functional groups in terms of chemical structures, properties and reactions. Identify aliphatic alcohols experimentally. Select the appropriate reagents to dierentiate between alcohols and other organic compounds. Theoretical Background/Context - Aliphatic alcohols are non-aromatic hydrocarbons possessing at least one hydroxyl group within their structure. They can be either cyclic or acyclic compounds. Alcohols are considered to be neutral compounds. Aliphatic Cyclic Alcohol Aliphatic Acyclic Alcohol - Alcohols are also classied to primary, secondary and tertiary alcohols according to the number of carbon atoms attached to the carbon atom linked to the hydroxyl group. First: Preparation of Aliphatic Alcohols - Alcohols can be prepared through some chemical routes such as reduction of the corresponding aldehydes and ketones using some reducing agents such as lithium aluminum hydride or sodium borohydride. They can be also obtained from hydration of the corresponding alkenes. Some primary alcohols can be synthesized through the nucleophilic substitution of corresponding alkyl halides using potassium or sodium hydroxides. - Alcohols can be also obtained through some biological routes such as ethanol and butanol through fermentation processes in presence of glucose. Glucose is obtained from starch hydrolysis in the presence of yeast. 1 www.praxilabs.com Theoretical Background/Context (Cont’) Second: General Properties of Aliphatic Alcohols - Aliphatic alcohols are polar compounds and can form hydrogen bonds easily.