Decreased Biochemical Progression in Patients with Castration‑Resistant
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1 DECLARATION This Work Is Original and Has Not Been Previously
AKR1C3 inhibition by curcumin, demethoxycurcumin, and bisdemethoxycurcumin: an investigation Item Type Thesis or dissertation Authors Calhoon, Brecken Citation Calhoon, B. (2019). AKR1C3 inhibition by curcumin, demethoxycurcumin, and bisdemethoxycurcumin: an investigation. (Masters thesis). University of Chester, United Kingdom. Publisher University of Chester Rights Attribution-NonCommercial-NoDerivatives 4.0 International Download date 25/09/2021 00:32:38 Item License http://creativecommons.org/licenses/by-nc-nd/4.0/ Link to Item http://hdl.handle.net/10034/623327 1 DECLARATION This work is original and has not been previously submitted in support of a Degree qualification or other course. Signed ……Brecken Elizabeth Calhoon………. Date…02/10/2019….. Word count: 4188 2 Research Article AKR1C3 inhibition by curcumin, demethoxycurcumin, and bisdemethoxycurcumin: an investigation Brecken Calhoon Abstract Background: Aldo-keto reductase 1C3 (AKR1C3) has been shown to be overexpressed in cancers due to its regulatory roles in cell proliferation and differentiation. Curcumin, known to have anti-tumour properties, and its analogues demethoxycurcumin (DMC) and bisdemethoxycurcumin (BDMC) were studied to determine their inhibitory effects on the AKR1C3 enzyme. Methods: AKR1C3 was purified and analysed to determine its protein concentration in transformed Escherichia coli (E. coli) cells. Enzyme assays equaling 1 mL contained 2.82 mg/mL AKR1C3, 50 μM of 3 mM NADPH, and varying volumes of potassium phosphate (50 mM, pH 6.5), 9,10-phenanthrenequinone (PQ), and inhibitors were measured at 340 nm. The Vmax, Km, KI, and 2 of AKR1C3 in the presence and absence of inhibitors were determined using a non- linear regression analysis on Fig.P Software. Results: PQ alone found Vmax = 0.47 IU/mg, Km = .435 μM, Ki = 6.29 μM, and 2 = 0.946. -

Supplementary Table S4. FGA Co-Expressed Gene List in LUAD
Supplementary Table S4. FGA co-expressed gene list in LUAD tumors Symbol R Locus Description FGG 0.919 4q28 fibrinogen gamma chain FGL1 0.635 8p22 fibrinogen-like 1 SLC7A2 0.536 8p22 solute carrier family 7 (cationic amino acid transporter, y+ system), member 2 DUSP4 0.521 8p12-p11 dual specificity phosphatase 4 HAL 0.51 12q22-q24.1histidine ammonia-lyase PDE4D 0.499 5q12 phosphodiesterase 4D, cAMP-specific FURIN 0.497 15q26.1 furin (paired basic amino acid cleaving enzyme) CPS1 0.49 2q35 carbamoyl-phosphate synthase 1, mitochondrial TESC 0.478 12q24.22 tescalcin INHA 0.465 2q35 inhibin, alpha S100P 0.461 4p16 S100 calcium binding protein P VPS37A 0.447 8p22 vacuolar protein sorting 37 homolog A (S. cerevisiae) SLC16A14 0.447 2q36.3 solute carrier family 16, member 14 PPARGC1A 0.443 4p15.1 peroxisome proliferator-activated receptor gamma, coactivator 1 alpha SIK1 0.435 21q22.3 salt-inducible kinase 1 IRS2 0.434 13q34 insulin receptor substrate 2 RND1 0.433 12q12 Rho family GTPase 1 HGD 0.433 3q13.33 homogentisate 1,2-dioxygenase PTP4A1 0.432 6q12 protein tyrosine phosphatase type IVA, member 1 C8orf4 0.428 8p11.2 chromosome 8 open reading frame 4 DDC 0.427 7p12.2 dopa decarboxylase (aromatic L-amino acid decarboxylase) TACC2 0.427 10q26 transforming, acidic coiled-coil containing protein 2 MUC13 0.422 3q21.2 mucin 13, cell surface associated C5 0.412 9q33-q34 complement component 5 NR4A2 0.412 2q22-q23 nuclear receptor subfamily 4, group A, member 2 EYS 0.411 6q12 eyes shut homolog (Drosophila) GPX2 0.406 14q24.1 glutathione peroxidase -

A Common Analgesic Enhances the Anti-Tumour Activity of 5-Aza-2’- Deoxycytidine Through Induction of Oxidative Stress
bioRxiv preprint doi: https://doi.org/10.1101/2020.03.31.017947; this version posted April 1, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. A common analgesic enhances the anti-tumour activity of 5-aza-2’- deoxycytidine through induction of oxidative stress Hannah J. Gleneadie1,10, Amy H. Baker1, Nikolaos Batis2, Jennifer Bryant2, Yao Jiang3, Samuel J.H. Clokie4, Hisham Mehanna2, Paloma Garcia5, Deena M.A. Gendoo6, Sally Roberts5, Alfredo A. Molinolo7, J. Silvio Gutkind8, Ben A. Scheven1, Paul R. Cooper1, Farhat L. Khanim9 and Malgorzata Wiench1, 5,*. 1School of Dentistry, Institute of Clinical Studies, College of Medical and Dental Sciences, The University of Birmingham, Birmingham, B5 7EG, UK; 2Institute of Head and Neck Studies and Education (InHANSE), The University of Birmingham, Birmingham, B15 2TT, UK; 3School of Biosciences, The University of Birmingham, Birmingham, B15 2TT, UK; 4West Midlands Regional Genetics Laboratory, Birmingham Women’s and Children’s Hospital, Birmingham, B15 2TG, UK; 5Institute of Cancer and Genomic Sciences, College of Medical and Dental Sciences, The University of Birmingham, Birmingham, B15 2TT, UK; 6Centre for Computational Biology, Institute of Cancer and Genomic Sciences, The University of Birmingham, Birmingham, B15 2TT, UK; 7Moores Cancer Center and Department of Pathology, University of California San Diego, La Jolla, CA 92093, USA; 8Department of Pharmacology and Moores Cancer -

Influence of Indomethacin on Steroid Metabolism
H OH metabolites OH Article Influence of Indomethacin on Steroid Metabolism: Endocrine Disruption and Confounding Effects in Urinary Steroid Profiling of Anti-Doping Analyses Anna Stoll 1 , Michele Iannone 2 , Giuseppina De Gregorio 2, Francesco Molaioni 2, Xavier de la Torre 2 , Francesco Botrè 2,3 and Maria Kristina Parr 1,* 1 Institute of Pharmacy (Pharmaceutical and Medical Chemistry), Freie Universität Berlin, 14195 Berlin, Germany; [email protected] 2 Laboratorio Antidoping Federazione Medico Sportiva Italiana, 00197 Rome, Italy; [email protected] (M.I.); [email protected] (G.D.G.); [email protected] (F.M.); [email protected] (X.d.l.T.); [email protected] (F.B.) 3 Synathlon—Quartier Centre, ISSUL—Institut des Sciences du Sport, Université de Lausanne, 1015 Lausanne, Switzerland * Correspondence: [email protected]; Tel.: +49-30-838-51471 Received: 13 September 2020; Accepted: 9 November 2020; Published: 14 November 2020 Abstract: Anabolic androgenic steroids (AAS) are prohibited as doping substances in sports by the World Anti-Doping Agency. Concentrations and concentration ratios of endogenous AAS (steroid profile markers) in urine samples collected from athletes are used to detect their administration. Certain (non-prohibited) drugs have been shown to influence the steroid profile and thereby sophisticate anti-doping analysis. It was shown in vitro that the non-steroidal anti-inflammatory drug (NSAID) indomethacin inhibits selected steroid-biotransformations catalyzed by the aldo-keto reductase (AKR) 1C3, which plays a key role in the endogenous steroid metabolism. Kinetic parameters for the indomethacin-mediated inhibition of the AKR1C3 catalyzed reduction in etiocholanolone were determined in vitro using two comparing methods. -

Allen Gao, M.D., Ph.D
Allen Gao, M.D., Ph.D. Research/Academic Interests Dr. Gao’s research interests focus on understanding molecular mechanisms associated with progression of castration-resistant prostate cancer and metastasis to bone with the goal of identification of potential therapeutic targets for prostate cancer. Particular emphasis includes microRNAs, aberrant androgen receptor activation by cytokines and transcriptional factors such as Stat3 and NF-kB, targeting cell signaling pathways (AR, IL-6 and Stat3), and mechanisms of drug resistance in prostate cancer. Dr. Gao’s lab was among the first to find that selenium regulates androgen signaling and that IL-4 activates the androgen receptor mediated by NF-B and Stat6, made the discovery that IL-6 signaling in prostate cancer involves Stat3, defined Stat3 and NF-B interactions in prostate cancer, discovered niclosamide as a novel inhibitor of androgen receptor variants (AR-V7), and identified several novel resistance mechanisms to enzalutamide/abiraterone/docetaxel including p52, IL- 6/Stat3, intracrine androgens/AKR1C3, and ABCB1. Dr. Gao has published over 100 peer-reviewed articles in the area of prostate cancer. His research findings such as the discovery of niclosamide as a novel inhibitor of androgen receptor variants have translated into several clinical trials (NCT02807805, NCT03123978) that test the combination treatments with current therapies to advanced resistant prostate cancer. Another recent discovery of indomethacin as an inhibitor of AKR1C3 and synergizing enzalutamide has also translated into clinical trial (NCT02935205) to test the combination treatments with current therapies to advanced resistant prostate cancer. Dr. Gao has served numerous NIH, NCI, DOD and VA merits, and American Cancer Society (ACS) review panels including SPORE and PPG study sections. -

Cancer Cell Targeting Using Nanoparticles
(19) & (11) EP 2 436 376 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 04.04.2012 Bulletin 2012/14 A61K 9/14 (2006.01) B82B 1/00 (2006.01) A61P 35/00 (2006.01) A61K 9/51 (2006.01) (2006.01) (2006.01) (21) Application number: 11186037.5 A61K 31/337 A61K 9/48 (22) Date of filing: 31.03.2008 (84) Designated Contracting States: (72) Inventors: AT BE BG CH CY CZ DE DK EE ES FI FR GB GR • Zale, Stephen E. HR HU IE IS IT LI LT LU LV MC MT NL NO PL PT Cambridge, MA 02142 (US) RO SE SI SK TR • Ali, Mir Mikkaram Designated Extension States: Woburn, MA 01801 (US) AL BA MK RS (74) Representative: Harris, Jennifer Lucy et al (30) Priority: 28.09.2007 US 976197 P Kilburn & Strode LLP 20 Red Lion Street (62) Document number(s) of the earlier application(s) in London WC1R 4PJ (GB) accordance with Art. 76 EPC: 08744752.0 / 2 136 788 Remarks: •This application was filed on 20-10-2011 as a (71) Applicant: Bind Biosciences, Inc. divisional application to the application mentioned Cambridge, MA 02139 (US) under INID code 62. •Claims filed after the date of receipt of the divisional application (Rule 68(4) EPC). (54) Cancer cell targeting using nanoparticles (57) The present invention generally relates to poly- having highly controlled properties, which can be formed mersand macromolecules, inparticular, to polymersuse- by mixing together two or more macromolecules in dif- ful in particles such as nanoparticles. -

1 Steroidogenic Enzyme AKR1C3 Is a Novel Androgen Receptor
Author Manuscript Published OnlineFirst on August 30, 2013; DOI: 10.1158/1078-0432.CCR-13-1151 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. Steroidogenic Enzyme AKR1C3 is a Novel Androgen Receptor-Selective Coactivator That Promotes Prostate Cancer Growth Muralimohan Yepuru, Zhongzhi Wu, Anand Kulkarni1, Feng Yin, Christina M. Barrett, Juhyun Kim, Mitchell S. Steiner, Duane D. Miller, James T. Dalton#* and Ramesh Narayanan#* Preclinical Research and Development, GTx Inc., Memphis, TN 1Department of Pathology, University of Tennessee Health Science Center, Memphis, TN. #RN & JTD share senior authorship. *Address correspondence to James T. Dalton ([email protected]) or Ramesh Narayanan ([email protected]) Preclinical Research and Development GTx Inc., 3 North Dunlap, Memphis, TN-38163 Ph. 901-523-9700 Fax. 901-523-9772 Conflict of interest: MY, ZW, FY, CMB, JK, MSS, DDM, JTD, and RN are employees of GTx, Inc. 1 Downloaded from clincancerres.aacrjournals.org on September 25, 2021. © 2013 American Association for Cancer Research. Author Manuscript Published OnlineFirst on August 30, 2013; DOI: 10.1158/1078-0432.CCR-13-1151 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. Translational Relevance Castration resistant prostate cancer (CRPC) is characterized by the emergence of a hypersensitive androgen signaling axis after orchiectomy or medical castration. The revival of androgen signaling is believed to be due to high intra-tumoral androgen synthesis, fueled by up-regulation of steroidogenic enzymes, including AKR1C3. Here, for the first time, using molecular and in vivo preclinical models, and human CRPC tissues, we demonstrate that the steroidogenic enzyme AKR1C3 also acts as a selective coactivator for androgen receptor to promote CRPC growth. -

MOL 6569 1 TITLE PAGE Development of Non-Steroidal Anti
Molecular Pharmacology Fast Forward. Published on October 8, 2004 as DOI: 10.1124/mol.104.006569 Molecular PharmacologyThis article has Fast not been Forward. copyedited Published and formatted. on The October final version 8, may 2004 differ as from doi:10.1124/mol.104.006569 this version. MOL 6569 TITLE PAGE Development of non-steroidal anti-inflammatory drug (NSAID) analogs and steroid carboxylates selective for human aldo-keto reductase isoforms: Potential antineoplastic agents that work independently of cyclooxygenase isozymes David R. Bauman, Steven Rudnick, Lawrence M. Szewczuk, Yi Jin, Sridhar Gopishetty, Downloaded from and Trevor M. Penning molpharm.aspetjournals.org Department of Pharmacology, University of Pennsylvania School of Medicine, Philadelphia, PA 19104-6084 at ASPET Journals on October 1, 2021 1 Copyright 2004 by the American Society for Pharmacology and Experimental Therapeutics. Molecular Pharmacology Fast Forward. Published on October 8, 2004 as DOI: 10.1124/mol.104.006569 This article has not been copyedited and formatted. The final version may differ from this version. MOL 6569 RUNNING TITLE PAGE Running title: NSAID analogs that target human AKR1Cs and not COX. To whom correspondence should be addressed: Dr. Trevor M. Penning, Department of Pharmacology, University of Pennsylvania School Downloaded from of Medicine, 130C John Morgan Building, 3620 Hamilton Walk, Philadelphia, PA 19104-6084, USA. Tel.: 215-898-9445; Fax.: 215-573-2236; E-mail: [email protected] molpharm.aspetjournals.org Number of text -
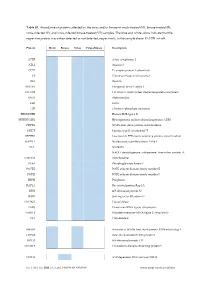
Virus-Infected (V), and Virus-Infected Binase-Treated (VB) Samples
Table S1. Abundance of proteins affected by the virus and/or binase in mock-treated (M), binase-treated (B), virus-infected (V), and virus-infected binase-treated (VB) samples. The blue and white colors indicate that the respective protein was either detected or not detected, respectively, in the sample above 1% FDR cut-off. Protein Mock Binase Virus Virus+Binase Description ACTB Actin, cytoplasmic 1 ATL2 Atlastin-2 CCT7 T-complex protein 1 subunit eta CS Citrate synthase, mitochondrial DES Desmin EEF1A1 Elongation factor 1-alpha 1 EFTUD2 116 kDa U5 small nuclear ribonucleoprotein component ENO1 Alpha-enolase EZR Ezrin GPI Glucose-6-phosphate isomerase HIST1H2BB Histone H2B type 1-B HNRNPA2B1 Heterogeneous nuclear ribonucleoproteins A2/B1 HSPD1 60 kDa heat shock protein, mitochondrial KRT75 Keratin, type II cytoskeletal 75 LRPPRC Leucine-rich PPR motif-containing protein, mitochondrial NAP1L1 Nucleosome assembly protein 1-like 1 NCL Nucleolin NADH dehydrogenase [ubiquinone] iron-sulfur protein 8, NDUFS8 mitochondrial PGK1 Phosphoglycerate kinase 1 POTEE POTE ankyrin domain family member E POTEI POTE ankyrin domain family member I PRPH Peripherin RAP1A Ras-related protein Rap-1A RPS2 40S ribosomal protein S2 SF3B1 Splicing factor 3B subunit 1 TALDO1 Transaldolase TARS Threonine--tRNA ligase, cytoplasmic TARSL2 Probable threonine--tRNA ligase 2, cytoplasmic TKT Transketolase AHSA1 Activator of 90 kDa heat shock protein ATPase homolog 1 HSPA2 Heat shock-related 70 kDa protein 2 RPL15 60S ribosomal protein L15 TXNDC5 Thioredoxin domain-containing protein 5 DDX18 ATP-dependent RNA helicase DDX18 Int. J. Mol. Sci. 2020, 21, x; doi: FOR PEER REVIEW www.mdpi.com/journal/ijms Int. -

The Common Analgesic Paracetamol Enhances the Anti-Tumour Activity of Decitabine Through Exacerbation of Oxidative Stress
Supplementary information for: The common analgesic paracetamol enhances the anti-tumour activity of decitabine through exacerbation of oxidative stress Hannah J. Gleneadie, Amy Baker, Nikolaos Batis, Jennifer Bryant, Yao Jiang, Samuel J.H. Clokie, Hisham Mehanna, Paloma Garcia, Deena M.A. Gendoo, Sally Roberts, Alfredo A. Molinolo, J. Silvio Gutkind, Ben A. Scheven, Paul R. Cooper, Farhat L. Khanim, Malgorzata Wiench. The Appendix contains the following supplementary information: SI Tables S1 to S7 (Tables S3 and S4 as separate .xlsx files) SI Figures S1 to S7 1 SI Supplementary Tables Table S1. DRI (Dose Reduction Index) for VU40T, combined treatment. VU40T Fa Dose DRI (%) DAC Para DAC Para 10 0.01 105 0.30 10.18 25 0.16 251 1.22 7.11 50 2.26 595 4.99 4.97 75 31 1414 20.35 3.47 97 9300 9200 426 1.59 Table S2. DRI for HN12, combined treatment. HN12 Fa Dose DRI (%) DAC Para DAC Para 10 0.004 186 0.49 80 25 0.06 1962 1.19 147 50 0.85 20653 2.92 269 75 11.92 217412 7.15 493 97 3630 3.55E+07 49.5 1827 2 Table S3. Differentially expressed (increased and decreased) genes in VU40T cells following DAC, paracetamol and DAC+paracetamol treatments. See the Table_S3.xlsx file. Table S4. Full list of GO terms from REVIGO for differentially expressed groups (DAC, paracetamol and DAC+paracetamol treatments). See the Table_S4.xlsx file. 3 Table S5. cBioPortal RNA-seq data (for cancers with provisional TCGA data): frequency of expression alterations (%) in genes from COX-2-PGE2 pathway and correlation to survival (Logrank test p-value). -

AKR1C3 Inhibitor KV-37 Exhibits Antineoplastic Effects And
Published OnlineFirst June 11, 2018; DOI: 10.1158/1535-7163.MCT-17-1023 Small Molecule Therapeutics Molecular Cancer Therapeutics AKR1C3 Inhibitor KV-37 Exhibits Antineoplastic Effects and Potentiates Enzalutamide in Combination Therapy in Prostate Adenocarcinoma Cells Kshitij Verma1, Nehal Gupta1, Tianzhu Zang2, Phumvadee Wangtrakluldee2, Sanjay K. Srivastava1,3, Trevor M. Penning2, and Paul C. Trippier1,4 Abstract Aldo-keto reductase 1C3 (AKR1C3), also known as type 5 sensitizes CRPC cell lines (22Rv1 and LNCaP1C3) toward the 17 b-hydroxysteroid dehydrogenase, is responsible for intra- antitumor effects of enzalutamide. Crucially, KV-37 does not tumoral androgen biosynthesis, contributing to the develop- induce toxicity in nonmalignant WPMY-1 prostate cells ment of castration-resistant prostate cancer (CRPC) and even- nor does it induce weight loss in mouse xenografts. Moreover, tual chemotherapeutic failure. Significant upregulation of KV-37 reduces androgen receptor (AR) transactivation and AKR1C3 is observed in CRPC patient samples and derived prostate-specific antigen expression levels in CRPC cell lines CRPC cell lines. As AKR1C3 is a downstream steroidogenic indicative of a therapeutic effect in prostate cancer. Combina- enzyme synthesizing intratumoral testosterone (T) and 5a- tion studies of KV-37 with enzalutamide reveal a very high dihydrotestosterone (DHT), the enzyme represents a promis- degree of synergistic drug interaction that induces significant ing therapeutic target to manage CRPC and combat the emer- reduction in prostate -

The University of Bradford Institutional Repository
Potent and selective aldo-keto reductase 1C3 (AKR1C3) inhibitors based on the benzoisoxazole moiety: application of a bioisosteric scaffold hopping approach to flufenamic acid Item Type Article Authors Pippione, A.C.; Carnovale, I.M.; Bonanni, D.; Sini, Marcella; Goyal, P.; Marini, E.; Pors, Klaus; Adinolfi, S.; Zonari, D.; Festuccia, C.; Wahlgren, W.Y.; Friemann, R.; Bagnati, R.; Boschi, D.; Oliaro- Bosso, S.; Lolli, M.L. Citation Pippione AC, Carnovale IM, Bonanni D et al (2018) Potent and selective aldo-keto reductase 1C3 (AKR1C3) inhibitors based on the benzoisoxazole moiety: application of a bioisosteric scaffold hopping approach to flufenamic acid. European Journal of Medicinal Chemistry. 150: 930-945. Rights © 2018 Elsevier. Reproduced in accordance with the publisher's self-archiving policy. This manuscript version is made available under the CC-BY-NC-ND 4.0 license. Download date 27/09/2021 03:13:51 Link to Item http://hdl.handle.net/10454/16082 The University of Bradford Institutional Repository http://bradscholars.brad.ac.uk This work is made available online in accordance with publisher policies. Please refer to the repository record for this item and our Policy Document available from the repository home page for further information. To see the final version of this work please visit the publisher’s website. Access to the published online version may require a subscription. Link to publisher’s version: https://doi.org/10.1016/j.ejmech.2018.03.040 Citation: Pippione AC, Carnovale IM, Bonanni D et al (2018) Potent and selective aldo-keto reductase 1C3 (AKR1C3) inhibitors based on the benzoisoxazole moiety: application of a bioisosteric scaffold hopping approach to flufenamic acid.