Preoperative Or Perioperative Docetaxel, Oxaliplatin, And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Faculty of Social and Behavioural Sciences
Display copy - Inkijkexemplaar Partner universities of the Faculty of Social and Behavioural Sciences Cultural Anthropology and Development Sociology Copenhagen University (Denmark) University of Tromso (Norway) University College London (UK) Université René Descartes, Parijs (France) Freie Universität, Berlin (Germany) Ludwig-Maximilians Universität, München (Germany) Ruprecht-Karls Universität, Heidelberg (Germany) Education and Child Studies Katholieke Universiteit Leuven (Belgium) Roskilde University (Denmark) Aarhus Universitet (via University college) (Denmark) Universität Trier (Germany) Universität Kassel (Germany) Karl Franzens Universität (Austria) Koç University (Turkey) Yasar University (Turkey) Şehir University (Turkey) Political Science University of Antwerp (Belgium) Universite Libre de Bruxelles (Belgium) Ruprecht-Karls-Universität (Germany) Universität Konstanz (Germany) Institut d'Etudes Politiques de Grenoble (France) National University of Ireland (Ireland) Universita di Bologna (Italy) Universitet I Bergen (Norway) University of Oslo (Norway) Charles University (Czech Republic) Bilkent University (Turkey) Bogazici University (Turkey) Aberystwyth University (UK) Raadpleeg BB-pagina Study Abroad Political Science voor het actuele aanbod. Partner universities mentioned on this list are subject to change. No rights may be derived from the information above. Display copy - Inkijkexemplaar Psychology Universität Wien (Vienna, Austria) Universiteit Gent (Gent, Belgium) KU Leuven (Leuven, Belgium) Charles University (Prague, -

The Temperature-Dependent Effectiveness of Platinum-Based
cells Article The Temperature-Dependent Effectiveness of Platinum-Based Drugs Mitomycin-C and 5-FU during Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Colorectal Cancer Cell Lines Roxan F.C.P.A. Helderman 1,2 , Daan R. Löke 2, Jan Verhoeff 3 , Hans M. Rodermond 1, Gregor G.W. van Bochove 1, Menno Boon 1, Sanne van Kesteren 1, Juan J. Garcia Vallejo 3, H. Petra Kok 2, Pieter J. Tanis 4 , Nicolaas A.P. Franken 1,2 , Johannes Crezee 2 and Arlene L. Oei 1,2,* 1 Laboratory for Experimental Oncology and Radiobiology (LEXOR), Center for Experimental and Molecular Medicine (CEMM), Amsterdam University Medical Centers (UMC), University of Amsterdam, Cancer Center Amsterdam, P.O. Box 22700, 1100 DE Amsterdam, The Netherlands; [email protected] (R.F.C.P.A.H.); [email protected] (H.M.R.); [email protected] (G.G.W.v.B.); [email protected] (M.B.); [email protected] (S.v.K.); [email protected] (N.A.P.F.) 2 Department of Radiation Oncology, Amsterdam UMC, University of Amsterdam, P.O. Box 22700, 1100 DE Amsterdam, The Netherlands; [email protected] (D.R.L.); [email protected] (H.P.K.); [email protected] (J.C.) 3 Department of Molecular Cell Biology & Immunology, Amsterdam Infection & Immunity Institute and Cancer Center Amsterdam, Amsterdam UMC, P.O. Box 7057, 1007 MB Amsterdam, The Netherlands; j.verhoeff@amsterdamumc.nl (J.V.); [email protected] (J.J.G.V.) 4 Department for Surgery, Amsterdam UMC, University of Amsterdam, Cancer Center Amsterdam, P.O. -

Progression of Brain Atrophy in Spinocerebellar Ataxia Type 2: a Longitudinal Tensor- Based Morphometry Study
Progression of Brain Atrophy in Spinocerebellar Ataxia Type 2: A Longitudinal Tensor- Based Morphometry Study The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Mascalchi, M., S. Diciotti, M. Giannelli, A. Ginestroni, A. Soricelli, E. Nicolai, M. Aiello, et al. 2014. “Progression of Brain Atrophy in Spinocerebellar Ataxia Type 2: A Longitudinal Tensor-Based Morphometry Study.” PLoS ONE 9 (2): e89410. doi:10.1371/journal.pone.0089410. http://dx.doi.org/10.1371/ journal.pone.0089410. Published Version doi:10.1371/journal.pone.0089410 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:12064527 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA Progression of Brain Atrophy in Spinocerebellar Ataxia Type 2: A Longitudinal Tensor-Based Morphometry Study Mario Mascalchi1,2*, Stefano Diciotti2,3, Marco Giannelli4, Andrea Ginestroni5, Andrea Soricelli6,7, Emanuele Nicolai6, Marco Aiello6, Carlo Tessa8, Lucia Galli9, Maria Teresa Dotti10, Silvia Piacentini11, Elena Salvatore12, Nicola Toschi13,14,15 1 Quantitative and Functional Neuroradiology Research Program at Meyer Children Hospital and Careggi General Hospital, Florence, Italy, 2 ‘‘Mario Serio’’ Department of Experimental and Clinical Biomedical Sciences, University of Florence, Florence, Italy, -

Table of Contents
Table of Contents Welcome Message from the General Chair.......................................................... 2 M&N 2019 Organizers .......................................................................................... 3 Sponsors .............................................................................................................. 6 Patrons ................................................................................................................. 7 Exhibitors ............................................................................................................ 10 Monday, July 8 ....................................................................................................12 Tuesday, July 9 ................................................... ................................................19 Wednesday, July 10 ........................................................................................... 26 1 Welcome Message from the General Co-Chairs Dear colleagues and friends, On behalf of the entire Conference Committee, we are pleased to welcome you to the 5th IEEE International Symposium on Measurements and Networking (M&N 2019), which is held in Catania and hosted in Museo Diocesano in the heart of the city. The Symposium is mainly promoted by the IEEE IMS TC-37 Measurements and Networking, the IEEE IM Italy Chapter and by the IEEE Italy Section Systems Council Chapter. IEEE M&N is a privileged forum for the discussion of current and emerging trends on measurements, communications, computer science, -
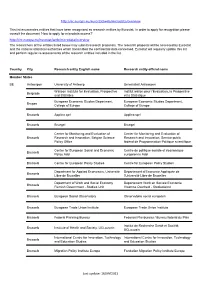
Eurostat: Recognized Research Entity
http://ec.europa.eu/eurostat/web/microdata/overview This list enumerates entities that have been recognised as research entities by Eurostat. In order to apply for recognition please consult the document 'How to apply for microdata access?' http://ec.europa.eu/eurostat/web/microdata/overview The researchers of the entities listed below may submit research proposals. The research proposal will be assessed by Eurostat and the national statistical authorities which transmitted the confidential data concerned. Eurostat will regularly update this list and perform regular re-assessments of the research entities included in the list. Country City Research entity English name Research entity official name Member States BE Antwerpen University of Antwerp Universiteit Antwerpen Walloon Institute for Evaluation, Prospective Institut wallon pour l'Evaluation, la Prospective Belgrade and Statistics et la Statistique European Economic Studies Department, European Economic Studies Department, Bruges College of Europe College of Europe Brussels Applica sprl Applica sprl Brussels Bruegel Bruegel Center for Monitoring and Evaluation of Center for Monitoring and Evaluation of Brussels Research and Innovation, Belgian Science Research and Innovation, Service public Policy Office fédéral de Programmation Politique scientifique Centre for European Social and Economic Centre de politique sociale et économique Brussels Policy Asbl européenne Asbl Brussels Centre for European Policy Studies Centre for European Policy Studies Department for Applied Economics, -

Testicular Cancer Treatment Regimens
Testicular Cancer Treatment Regimens Clinical Trials: The NCCN recommends cancer patient participation in clinical trials as the gold standard for treatment. Cancer therapy selection, dosing, administration, and the management of related adverse events can be a complex process that should be handled by an experienced healthcare team. Clinicians must choose and verify treatment options based on the individual patient; drug dose modifications and supportive care interventions should be administered accordingly. The cancer treatment regimens below may include both U.S. Food and Drug Administration-approved and unapproved indications/regimens. These regimens are only provided to supplement the latest treatment strategies. These Guidelines are a work in progress that may be refined as often as new significant data becomes available. The National Comprehensive Cancer Network Guidelines® are a consensus statement of its authors regarding their views of currently accepted approaches to treatment. Any clinician seeking to apply or consult any NCCN Guidelines® is expected to use independent medical judgment in the context of individual clinical circumstances to determine any patient’s care or treatment. The NCCN makes no warranties of any kind whatsoever regarding their content, use, or application and disclaims any responsibility for their application or use in any way. Note: All recommendations are category 2A unless otherwise indicated. uPrimary Chemotherapy for Germ Cell Tumors1 REGIMEN DOSING Preferred Regimens BEP (Bleomycin + Etoposide + Days 1-5: Cisplatin 20mg/m2 IV over 60 minutes dailya Cisplatin)2,a,b Days 1-5: Etoposide 100mg/m2 IV over 60 minutes daily Days 1,8,15 OR Days 2,9,16: Bleomycin 30 units IV over 10 minutes daily. -

The Role of ABCG2 in Modulating Responses to Anti-Cancer Photodynamic Therapy
This is a repository copy of The role of ABCG2 in modulating responses to anti-cancer photodynamic therapy. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/152665/ Version: Accepted Version Article: Khot, MI orcid.org/0000-0002-5062-2284, Downey, CL, Armstrong, G et al. (4 more authors) (2020) The role of ABCG2 in modulating responses to anti-cancer photodynamic therapy. Photodiagnosis and Photodynamic Therapy, 29. 101579. ISSN 1572-1000 https://doi.org/10.1016/j.pdpdt.2019.10.014 © 2019 Elsevier B.V. All rights reserved. This manuscript version is made available under the CC-BY-NC-ND 4.0 license http://creativecommons.org/licenses/by-nc-nd/4.0/. Reuse This article is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs (CC BY-NC-ND) licence. This licence only allows you to download this work and share it with others as long as you credit the authors, but you can’t change the article in any way or use it commercially. More information and the full terms of the licence here: https://creativecommons.org/licenses/ Takedown If you consider content in White Rose Research Online to be in breach of UK law, please notify us by emailing [email protected] including the URL of the record and the reason for the withdrawal request. [email protected] https://eprints.whiterose.ac.uk/ The role of ABCG2 in modulating responses to anti-cancer photodynamic therapy List of Authors: M. Ibrahim Khot, Candice L. Downey, Gemma Armstrong, Hafdis S. Svavarsdottir, Fazain Jarral, Helen Andrew and David G. -

Print Special Issue Flyer
IMPACT CITESCORE FACTOR 2.4 2.847 SCOPUS an Open Access Journal by MDPI Anaerobic Digestion Processes Guest Editors: Message from the Guest Editors Dr. Pietro Bartocci This Special Issue on "Anaerobic Digestion Processes" aims CRB Italian Biomass Research to curate novel advances in biogas production and use, Centre, University of Perugia, focusing both on modeling and experimental campaigns. 06100 Perugia, Italy Topics include, but are not limited to, the following: [email protected] Modeling of anaerobic digestion and biogas Prof. Qing Yang John A. Paulson School of production; Engineering and Applied Organic substrate characterization and pre- Sciences, Harvard University, treatment; Cambridge, MA 02138, USA Biogas purification and biomethane production [email protected] and use; Biogas combustion in engines and turbines. Prof. Francesco Fantozzi Department of Industrial Engineering, University of Perugia, Via G. Duranti 67, 06125 Perugia, Italy [email protected] Deadline for manuscript submissions: 30 November 2021 mdpi.com/si/30853 SpeciaIslsue IMPACT CITESCORE FACTOR 2.4 2.847 SCOPUS an Open Access Journal by MDPI Editor-in-Chief Message from the Editor-in-Chief Prof. Dr. Giancarlo Cravotto Processes (ISSN 2227-9717) provides an advanced forum Department of Drug Science and for process/system-related research in chemistry, biology, Technology, University of Turin, material, energy, environment, food, pharmaceutical, Via P. Giuria 9, 10125 Turin, Italy manufacturing and allied engineering fields. The journal publishes regular research papers, communications, letters, short notes and reviews. Our aim is to encourage researchers to publish their experimental, theoretical and computational results in as much detail as necessary. There is no restriction on paper length or number of figures and tables. -

Pier Giovanni Bissiri – Curriculum Vitae
Department of Statistical Sciences "Paolo Fortunati" Via Belle Arti 41 40126 Bologna Pier Giovanni Bissiri T +39 0512098279 B [email protected] Curriculum Vitae ORCID: https://orcid.org/0000-0003-3769-6649 Present position 11/2019– Tenure–track assistant professor (senior), at Department of Statistical Sciences, University of Bologna, Bologna, Italy. Previous positions 10/2017–11/2019 Research associate, at School of Mathematics, Statistics and Physics, New- castle University, Newcastle, UK, supervisor: prof. Emilio Porcu . topic: Positive definite functions in geostatistics 01/2015–09/2017 Postdoctoral position (renewed on 01/01/2017), at the Dep. of Econo- mics, Management and Statistics, University of Milano–Bicocca, Milan, Italy, supervisor: prof. A. Ongaro. topic: discrete Bayesian nonparametric models 04/2014–12/2014 Postdoctoral position, at IMATI, Institute of Applied Mathematics and Information Technology of the National Research Council (CNR), Milan, Italy. 01/2014–03/2014 Collaborator, at the Department of Economics, Management and Statistics of the University of Milano–Bicocca, Milan, Italy. 01/2010–12/2013 Postdoctoral position (renewed on 01/01/2012), at the Department of Economics, Management and Statistics of the University of Milano–Bicocca, Milan, Italy. topic: Non parametric Bayesian inference 12/2007–11/2009 Two years research grant ‘Master and Back’, funded by the Region of Sardinia, Italy, at the Department of Mathematics of the University of Cagliari, Italy. Qualification for professorship 10/02/2014 National scientific qualification to function as associate professor in Italian Universities in Statistics, obtained for the period 10/02/2014–10/02/2020, and renewed until 26/11/2020. https://asn.cineca.it/ministero.php/ public/esito/settore/13%252FD1/fascia/2 Education 19/1/2007 PhD in “Mathematics and Statistics”, Department of Mathematics at the University of Pavia, Pavia, Italy, Curriculum: Probability and Statistics. -

Creating Value in the Entrepreneurial University: Marketization and Merchandising Strategies
administrative sciences Article Creating Value in the Entrepreneurial University: Marketization and Merchandising Strategies Chiara Fantauzzi *, Rocco Frondizi , Nathalie Colasanti and Gloria Fiorani Department of Management and Law, University of Rome Tor Vergata, 00133 Roma, Italy; [email protected] (R.F.); [email protected] (N.C.); gloria.fi[email protected] (G.F.) * Correspondence: [email protected] Received: 9 August 2019; Accepted: 14 October 2019; Published: 18 October 2019 Abstract: Higher education institutions are called to expand their role and responsibilities, by enhancing their entrepreneurial mindset and redefining relationships with stakeholders. In order to cope with these new challenges, they have started to operate in a strategic manner, by performing marketing and merchandising activities. Indeed, in a sector characterized by the presence of competitive funding models, several forms of accountability, and performance indicators, universities have become open systems and have started to operate like enterprises, considering students as customers. Given this premise, the aim of the paper is to individuate marketing and merchandising strategies in higher education and to evaluate their effectiveness in order to foster stakeholders engagement. This is in line with the entrepreneurial university model that represents the starting point of the theoretical study, then a literature review of “marketization” in higher education institutions is presented, showing how this field is not yet completely investigated. Data refer to the Italian context and are analyzed through a qualitative method. Findings suggest that most Italian universities perform merchandising strategies, but currently there is not sufficient information to evaluate their effectiveness in higher education, it was only possible to make hypotheses. -

Fact Sheet Siena 2020-2021
Fact Sheet 2020-2021 INSTITUTIONAL DETAILS Name Università degli Studi di Siena Erasmus Code I SIENA 01 City Siena Country Italy Website of Institution http://www.unisi.it CONTACTS University of Siena International Relations Division Address Via San Vigilio, 6 53100 SIENA (Italy) Website for Exchange http://en.unisi.it/international/international-exchange-students Students Dr.ssa Annalisa POGGIALINI Responsible of the Email: [email protected] International Relations Tel: +39-0577-235028 Division Deputy Rector for Prof. Luca Verzichelli International Relations [email protected] Candida CALVO VICENTE - Elisa CAVICCHIOLI – Simona QUERCI Incoming Mobility Office Contact persons for Tel: +39 0577 235182 - 235123 - 235124 incoming students E-mail: [email protected] Contact persons for Tiziana GATTI – Simona CIANI – Anna PRATESI outgoing Erasmus + European Programs Office students and Erasmus + Tel. +39 0577 235199 - 235198 - 235201 inter-institutional E-mail: [email protected] agreements Milena FADDA – Laura ANGOTTI – Linda MORGESE – Piero PILLON Contact persons for Office of International Programmes outgoing Overseas Tel. +39 0577 235199 - 235198 - 235201 students and bilateral E-mail: [email protected] agreements The University of Siena will send students’ electronic transcripts of records to the Contact Office for email address communicated by the home University. transcript of records Giulia CARLI - Marco CARATELLI and confirmation of Tel. +39 0577 235121 - 235323 stay E-mail: [email protected] APPLICATION PROCEDURES Nomination Each University must send the students’ nominations through the Unisi Nomination Form. The Incoming Mobility Team will provide the partner Universities with the link to the nomination form. Due to the Covid-19 pandemic, we will be flexible with the deadline for Nomination Deadline student's nomination. -

BC Cancer Benefit Drug List September 2021
Page 1 of 65 BC Cancer Benefit Drug List September 2021 DEFINITIONS Class I Reimbursed for active cancer or approved treatment or approved indication only. Reimbursed for approved indications only. Completion of the BC Cancer Compassionate Access Program Application (formerly Undesignated Indication Form) is necessary to Restricted Funding (R) provide the appropriate clinical information for each patient. NOTES 1. BC Cancer will reimburse, to the Communities Oncology Network hospital pharmacy, the actual acquisition cost of a Benefit Drug, up to the maximum price as determined by BC Cancer, based on the current brand and contract price. Please contact the OSCAR Hotline at 1-888-355-0355 if more information is required. 2. Not Otherwise Specified (NOS) code only applicable to Class I drugs where indicated. 3. Intrahepatic use of chemotherapy drugs is not reimbursable unless specified. 4. For queries regarding other indications not specified, please contact the BC Cancer Compassionate Access Program Office at 604.877.6000 x 6277 or [email protected] DOSAGE TUMOUR PROTOCOL DRUG APPROVED INDICATIONS CLASS NOTES FORM SITE CODES Therapy for Metastatic Castration-Sensitive Prostate Cancer using abiraterone tablet Genitourinary UGUMCSPABI* R Abiraterone and Prednisone Palliative Therapy for Metastatic Castration Resistant Prostate Cancer abiraterone tablet Genitourinary UGUPABI R Using Abiraterone and prednisone acitretin capsule Lymphoma reversal of early dysplastic and neoplastic stem changes LYNOS I first-line treatment of epidermal