Novel Treatments for Type 2 Diabetes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dualism of Peroxisome Proliferator-Activated Receptor Α/Γ: a Potent Clincher in Insulin Resistance
AEGAEUM JOURNAL ISSN NO: 0776-3808 Dualism of Peroxisome Proliferator-Activated Receptor α/γ: A Potent Clincher in Insulin Resistance Mr. Ravikumar R. Thakar1 and Dr. Nilesh J. Patel1* 1Faculty of Pharmacy, Shree S. K. Patel College of Pharmaceutical Education & Research, Ganpat University, Gujarat, India. [email protected] Abstract: Diabetes mellitus is clinical syndrome which is signalised by augmenting level of sugar in blood stream, which produced through lacking of insulin level and defective insulin activity or both. As per worldwide epidemiology data suggested that the numbers of people with T2DM living in developing countries is increasing with 80% of people with T2DM. Peroxisome proliferator-activated receptors are a family of ligand-activated transcription factors; modulate the expression of many genes. PPARs have three isoforms namely PPARα, PPARβ/δ and PPARγ that play a central role in regulating glucose, lipid and cholesterol metabolism where imbalance can lead to obesity, T2DM and CV ailments. It have pathogenic role in diabetes. PPARα is regulates the metabolism of lipids, carbohydrates, and amino acids, activated by ligands such as polyunsaturated fatty acids, and drugs used as Lipid lowering agents. PPAR β/δ could envision as a therapeutic option for the correction of diabetes and a variety of inflammatory conditions. PPARγ is well categorized, an element of the PPARs, also pharmacological effective as an insulin resistance lowering agents, are used as a remedy for insulin resistance integrated with type- 2 diabetes mellitus. There are mechanistic role of PPARα, PPARβ/δ and PPARγ in diabetes mellitus and insulin resistance. From mechanistic way, it revealed that dual PPAR-α/γ agonist play important role in regulating both lipids as well as glycemic levels with essential safety issues. -

Diabetes Mellitus Typ 2 Medikamentöse Therapie
Übersicht AMT Diabetes mellitus Typ 2 Medikamentöse Therapie L. Cornelius Bollheimer, Christiane Girlich, Ulrike Woenckhaus und Roland Büttner, Regensburg Arzneimitteltherapie 2007;25:175–86. Literatur NIDDM subjects. A study of two ethnic groups. Diabetes Care 1993;16: 621–9. 1. Deutsche Diabetes Gesellschaft. Evidenzbasierte Leitlinie: Epidemiologie 24. Akbar DH. Effect of metformin and sulfonylurea on C-reactive protein und Verlauf des Diabetes mellitus in Deutschland. http://www.deutsche- level in well-controlled type 2 diabetics with metabolic syndrome. En- diabetes-gesellschaft.de/redaktion/mitteilungen/leitlinien/EBL_Update_ docrine 2003;20:215–8. Epidemiologie_05_2004_neues_Layout.pdf. Internetdokument. 2004. 25. Krentz AJ, Bailey CJ. Oral antidiabetic agents: current role in type 2 dia- 2. Seufert J. Kardiovaskuläre Endpunktstudien in der Therapie des Typ-2- betes mellitus. Drugs 2005;65:385–411. Diabetes-mellitus. Dtsch Ärzteblatt 2006;103:A934–42. 26. Parhofer KG, Laubach E, Geiss HC, Otto C. Effect of glucose control on 3. Deutsche Diabetes Gesellschaft. Praxis-Leitlinien der Deutschen Diabe- lipid levels in patients with type 2 diabetes. Dtsch Med Wochenschr tes Gesellschaft. Diabetologie und Stoffwechsel 2006;1:S2. 2002;127:958–62. 4. Häring HU, Matthaei S. Behandlung des Diabetes mellitus Typ 2. Diabe- 27. DeFronzo RA, Barzilai N, Simonson DC. Mechanism of metformin ac- tologie und Stoffwechsel 2006;1:S205–10. tion in obese and lean noninsulin-dependent diabetic subjects. J Clin 5. Brueckel J, Kerner W. Definition, Klassifikation und Diagnostik des Dia- Endocrinol Metab 1991;73:1294–301. betes mellitus. Diabetologie und Stoffwechsel 2006;1:S177–80. 28. Cryer DR, Nicholas SP, Henry DH, Mills DJ, et al. Comparative outcomes 6. -

Combination Treatment with Pioglitazone and Fenofibrate
179 Combination treatment with pioglitazone and fenofibrate attenuates pioglitazone-mediated acceleration of bone loss in ovariectomized rats Rana Samadfam, Malaika Awori, Agnes Be´nardeau1, Frieder Bauss2, Elena Sebokova1, Matthew Wright1 and Susan Y Smith Charles River Laboratories, 22022 Transcanadienne, Senneville, Montre´al, Que´bec, Canada H9X 3R3 1F. Hoffmann-La Roche AG, Basel, CH-4070 Switzerland 2Roche Diagnostics GmbH, Penzberg, DE-82377 Germany (Correspondence should be addressed to S Y Smith; Email: [email protected]) Abstract Peroxisome proliferator-activated receptor (PPAR) g ago- mineral content (w45%) and bone mineral density (BMD; nists, such as pioglitazone (Pio), improve glycemia and lipid w60%) at the lumbar spine. Similar effects of treatments were profile but are associated with bone loss and fracture risk. Data observed at the femur, most notably at sites rich in trabecular regarding bone effects of PPARa agonists (including bone. At the proximal tibial metaphysis, concomitant fenofibrate (Feno)) are limited, although animal studies treatment with PioCFeno prevented Pio exacerbation of suggest that Feno may increase bone mass. This study ovariectomy-induced loss of trabecular bone, resulting in investigated the effects of a 13-week oral combination BMD values in the PioCFeno group comparable to OVX treatment with Pio (10 mg/kg per day)CFeno (25 mg/kg controls. Discontinuation of Pio or Feno treatment of per day) on body composition and bone mass parameters OVX rats was associated with partial reversal of effects on compared with Pio or Feno alone in adult ovariectomized bone loss or bone mass gain, respectively, while values in the (OVX) rats, with a 4-week bone depletion period, followed PioCFeno group remained comparable to OVX controls. -

Comparative Transcriptional Network Modeling of Three PPAR-A/C Co-Agonists Reveals Distinct Metabolic Gene Signatures in Primary Human Hepatocytes
Comparative Transcriptional Network Modeling of Three PPAR-a/c Co-Agonists Reveals Distinct Metabolic Gene Signatures in Primary Human Hepatocytes Rene´e Deehan1, Pia Maerz-Weiss2, Natalie L. Catlett1, Guido Steiner2, Ben Wong1, Matthew B. Wright2*, Gil Blander1¤a, Keith O. Elliston1¤b, William Ladd1, Maria Bobadilla2, Jacques Mizrahi2, Carolina Haefliger2, Alan Edgar{2 1 Selventa, Cambridge, Massachusetts, United States of America, 2 F. Hoffmann-La Roche AG, Basel, Switzerland Abstract Aims: To compare the molecular and biologic signatures of a balanced dual peroxisome proliferator-activated receptor (PPAR)-a/c agonist, aleglitazar, with tesaglitazar (a dual PPAR-a/c agonist) or a combination of pioglitazone (Pio; PPAR-c agonist) and fenofibrate (Feno; PPAR-a agonist) in human hepatocytes. Methods and Results: Gene expression microarray profiles were obtained from primary human hepatocytes treated with EC50-aligned low, medium and high concentrations of the three treatments. A systems biology approach, Causal Network Modeling, was used to model the data to infer upstream molecular mechanisms that may explain the observed changes in gene expression. Aleglitazar, tesaglitazar and Pio/Feno each induced unique transcriptional signatures, despite comparable core PPAR signaling. Although all treatments inferred qualitatively similar PPAR-a signaling, aleglitazar was inferred to have greater effects on high- and low-density lipoprotein cholesterol levels than tesaglitazar and Pio/Feno, due to a greater number of gene expression changes in pathways related to high-density and low-density lipoprotein metabolism. Distinct transcriptional and biologic signatures were also inferred for stress responses, which appeared to be less affected by aleglitazar than the comparators. In particular, Pio/Feno was inferred to increase NFE2L2 activity, a key component of the stress response pathway, while aleglitazar had no significant effect. -

CDR Clinical Review Report for Soliqua
CADTH COMMON DRUG REVIEW Clinical Review Report Insulin glargine and lixisenatide injection (Soliqua) (Sanofi-Aventis) Indication: adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus inadequately controlled on basal insulin (less than 60 units daily) alone or in combination with metformin. Service Line: CADTH Common Drug Review Version: Final (with redactions) Publication Date: January 2019 Report Length: 118 Pages Disclaimer: The information in this document is intended to help Canadian health care decision-makers, health care professionals, health systems leaders, and policy-makers make well-informed decisions and thereby improve the quality of health care services. While patients and others may access this document, the document is made available for informational purposes only and no representations or warranties are made with respect to its fitness for any particular purpose. The information in this document should not be used as a substitute for professional medical advice or as a substitute for the application of clinical judgment in respect of the care of a particular patient or other professional judgment in any decision-making process. The Canadian Agency for Drugs and Technologies in Health (CADTH) does not endorse any information, drugs, therapies, treatments, products, processes, or services. While care has been taken to ensure that the information prepared by CADTH in this document is accurate, complete, and up-to-date as at the applicable date the material was first published by CADTH, CADTH does not make any guarantees to that effect. CADTH does not guarantee and is not responsible for the quality, currency, propriety, accuracy, or reasonableness of any statements, information, or conclusions contained in any third-party materials used in preparing this document. -

Download Product Insert (PDF)
PRODUCT INFORMATION Remogliflozin A Item No. 14340 CAS Registry No.: 329045-45-6 OH Formal Name: 5-methyl-4-[[4-(1-methylethoxy) N O OH phenyl]methyl]-1-(1-methylethyl)- N 1H-pyrazol-3-yl β-D- O glucopyranoside OH Synonym: GSK189074 OH MF: C23H34N2O7 FW: 450.5 Purity: ≥98% λ: 229 nm UV/Vis.: max O Supplied as: A crystalline solid Storage: -20°C Stability: ≥2 years Information represents the product specifications. Batch specific analytical results are provided on each certificate of analysis. Laboratory Procedures Remogliflozin A is supplied as a crystalline solid. A stock solution may be made by dissolving the remogliflozin A in the solvent of choice. Remogliflozin A is soluble in organic solvents such as ethanol, DMSO, and dimethyl formamide, which should be purged with an inert gas. The solubility of remogliflozin A in these solvents is approximately 30 mg/ml. Remogliflozin A is sparingly soluble in aqueous buffers. For maximum solubility in aqueous buffers, remogliflozin A should first be dissolved in ethanol and then diluted with the aqueous buffer of choice. Remogliflozin A has a solubility of approximately 0.5 mg/ml in a 1:1 solution of ethanol:PBS (pH 7.2) using this method. We do not recommend storing the aqueous solution for more than one day. Description Remogliflozin A is a potent inhibitor of sodium-glucose transporter 2 (SGLT2; Kis = 12.4 and 26 nM 1 for human and rat SGLT2, respectively). It is selective for SGLT2 over SGLT1 (Kis = 4,520 and 997 nM for human and rat SGLT1, respectively). Following administration of a prodrug, remogliflozin etabonate, that is rapidly converted to remogliflozin A in vivo, rat urinary glucose excretion increases and plasma glucose and insulin concentrations decrease. -

Lobeglitazone
2013 International Conference on Diabetes and Metabolism Lobeglitazone, A Novel PPAR-γ agonist with balanced efficacy and safety Kim, Sin Gon. MD, PhD. Professor, Division of Endocrinology and Metabolism Department of Internal Medicine, Korea University College of Medicine. Disclosure of Financial Relationships This symposium is sponsored by Chong Kun Dang Pharmaceutical Corp. I have received lecture and consultation fees from Chong Kun Dang. Pros & Cons of PPAR-γ agonist Pros Cons • Good glucose lowering • Adverse effects • Durability (ADOPT) (edema, weight gain, • Insulin sensitizing CHF, fracture or rare effects (especially in MS, macular edema etc) NAFLD, PCOS etc) • Possible safety issues • Prevention of new- (risk of MI? – Rosi or onset diabetes (DREAM, bladder cancer? - Pio) ACT-NOW) • LessSo, hypoglycemiathere is a need to develop PPAR-γ • Few GI troubles agonist• Outcome with data balanced efficacy and safety (PROactive) Insulin Sensitizers : Several Issues Rosi, Peak sale ($3.3 billion) DREAM Dr. Nissen Dr. Nissen ADOPT META analysis BARI-2D (5,8) Rosi, lipid profiles RECORD 1994 1997 1999 2000 2002 2004 2005 2006 2007 2008 2009 2010 2011 2012 2013 2014 Tro out d/t FDA, All diabetes hepatotoxicity drug CV safety Rosi (5) FDA, Black box Rosi, Rosi , CV safety warning - REMS in USA = no evidence - Europe out Pio (7) PIO, bladder cancer CKD 501 Lobeglitazone 2000.6-2004.6 2004.11-2007.1 2007.3-2008.10 2009.11-2011.04 Discovery& Preclinical study Phase I Phase II Phase III Developmental Strategy Efficacy • PPAR activity Discovery & Preclinical study • In vitro & vivo efficacy • Potent efficacy 2000.06 - 2004.06 Phase I 2004.11 - 2007.01 • In vitro screening • Repeated dose toxicity • Metabolites • Geno toxicity • Phase II CYP 450 • Reproductive toxicity 2007.03 - 2008.10 • DDI • Carcinogenic toxicity ADME Phase III Safety 2009.11 - 2011.04 CV Safety / (Bladder) Cancer / Liver Toxicity / Bone loss Lobeglitazone (Duvie) 1. -

Comparative Effectiveness, Safety, and Indications of Insulin Analogues in Premixed Formulations for Adults with Type 2 Diabetes
Comparative Effectiveness Review Number 14 Comparative Effectiveness, Safety, and Indications of Insulin Analogues in Premixed Formulations for Adults With Type 2 Diabetes This report is based on research conducted by the Johns Hopkins Evidence-based Practice Center (EPC) under contract to the Agency for Healthcare Research and Quality (AHRQ), Rockville, MD (Contract No. 290-02-0018). The findings and conclusions in this document are those of the authors, who are responsible for its contents; the findings and conclusions do not necessarily represent the views of AHRQ. Therefore, no statement in this report should be construed as an official position of the Agency for Healthcare Research and Quality or of the U.S. Department of Health and Human Services. This report is intended as a reference and not as a substitute for clinical judgment. Anyone who makes decisions concerning the provision of clinical care should consider this report in the same way as any medical reference and in conjunction with all other pertinent information. This report may be used, in whole or in part, as the basis for development of clinical practice guidelines and other quality enhancement tools, or as a basis for reimbursement and coverage policies. AHRQ or U.S. Department of Health and Human Services endorsement of such derivative products may not be stated or implied. Comparative Effectiveness Review Number 14 Comparative Effectiveness, Safety, and Indications of Insulin Analogues in Premixed Formulations for Adults With Type 2 Diabetes Prepared for: Agency for Healthcare Research and Quality U.S. Department of Health and Human Services 540 Gaither Road Rockville, MD 20850 www.ahrq.gov Contract No. -

Antidiabetic Drugs in NAFLD: the Accomplishment of Two Goals at Once?
pharmaceuticals Review Antidiabetic Drugs in NAFLD: The Accomplishment of Two Goals at Once? Matteo Tacelli , Ciro Celsa , Bianca Magro, Aurora Giannetti, Grazia Pennisi, Federica Spatola and Salvatore Petta * Sezione di Gastroenterologia e Epatologia, DiBiMIS, University of Palermo, 90127 Palermo, Italy; [email protected] (M.T.); [email protected] (C.C.); [email protected] (B.M.); [email protected] (A.G.); [email protected] (G.P.); [email protected] (F.S.) * Correspondence: [email protected], Tel.: +39-091-655-2170; Fax: +39-091-655-2156 Received: 17 October 2018; Accepted: 3 November 2018; Published: 8 November 2018 Abstract: Non-Alcoholic Fatty Liver Disease (NAFLD) is the most common cause of chronic liver disease in Western countries, accounting for 20–30% of general population and reaching a prevalence of 55% in patients with type 2 diabetes mellitus (T2DM). Insulin resistance plays a key role in pathogenic mechanisms of NAFLD. Many drugs have been tested but no medications have yet been approved. Antidiabetic drugs could have a role in the progression reduction of the disease. The aim of this review is to summarize evidence on efficacy and safety of antidiabetic drugs in patients with NAFLD. Metformin, a biguanide, is the most frequently used drug in the treatment of T2DM. To date 15 randomized controlled trials (RCTs) and four meta-analysis on the use of metformin in NAFLD are available. No significant improvement in histological liver fibrosis was shown, but it can be useful in the treatment of co-factors of NAFLD, like body weight, transaminase or cholesterol levels, and HbA1c levels. -

Remogliflozin Etabonate, a Selective Inhibitor of the Sodium-Glucose
Clinical Care/Education/Nutrition/Psychosocial Research BRIEF REPORT Remogliflozin Etabonate, a Selective Inhibitor of the Sodium-Glucose Transporter 2, Improves Serum Glucose Profiles in Type 1 Diabetes 1,2 3 SUNDER MUDALIAR, MD JUNE YE, PHD placebo (placebo), 2) mealtime insulin 1 3 DEBRA A. ARMSTRONG, BA, RN, CCRC ELIZABETH K. HUSSEY, PHARMD 2 3 injection + RE placebo (prandial insulin), ANNIE A. MAVIAN, MD DEREK J. NUNEZ, MD 3 3 1,2 ) placebo insulin injection + 50 mg RE (RE ROBIN O’CONNOR-SEMMES, PHD ROBERT R. HENRY, MD 3 3 50 mg), 4) placebo insulin injection + 150 PATRICIA K. MYDLOW, BS ROBERT L. DOBBINS, MD, PHD mg RE (RE 150 mg), and 5) placebo insulin injection + 500 mg RE (RE 500 mg). d fl Each individual received 75-g oral OBJECTIVES Remogli ozin etabonate (RE), an inhibitor of the sodium-glucose transporter glucose and identical meals during all 2, improves glucose profiles in type 2 diabetes. This study assessed safety, tolerability, pharma- cokinetics, and pharmacodynamics of RE in subjects with type 1 diabetes. treatment periods. Frequent samples were obtained for measurement of plasma RESEARCH DESIGN AND METHODSdTen subjects managed with continuous sub- glucose and insulin concentrations. Urine cutaneous insulin infusion were enrolled. In addition to basal insulin, subjects received five samples were collected for 24 h to assess randomized treatments: placebo, prandial insulin, 50 mg RE, 150 mg RE, and mg RE 500. creatinine clearance and glucose excre- d tion. Plasma samples were collected for RESULTS Adverse events and incidence of hypoglycemia with RE did not differ from placebo fl and prandial insulin groups. -
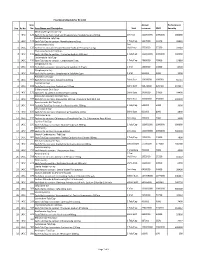
Sno. Rc No Item No. Item Name and Description Unit Annual Turnover
Final Drug Schedule for RC 145C Item Annual Performance Sno. Rc No No. Item Name and Description Unit turnover EMD Security Medroxy Progesterone Inj- 1 145C 126 Each ml to contain: Medroxy Progesterone Acetate Suspn.150mg. 1ml Vial 100000000 1000000 600000 Norethisterone Tab/Cap- 2 145C 128 Each Tab/Cap to contain: Norethisterone 5mg. 1 Tab/Cap 5637000 56370 33822 Dexamethasone Inj- 2ml 3 145C 133 Each ml to contain: Dexamethasone Sodium Phosphate 4 mg. Vial/Amp 5735000 57350 34410 Thyroxine Sodium Tab/Cap- 4 145C 135 Each Tab/Cap to contain: Thyroxine Sodium 100 mcg 1 Tab/Cap 100000000 1000000 600000 Carbimazole Tab/Cap- 5 145C 136 Each Tab/Cap to contain: Carbimazole 5 mg. 1 Tab/Cap 2980000 29800 17880 Streptomycin Inj- 6 145C 151 Each Vial to contain: Streptomycin Sulphate 0.75gm 1 Vial 1400000 14000 8400 Streptomycin Inj- 7 145C 152 Each Vial to contain: Streptomycin Sulphate 1gm 1 Vial 500000 5000 3000 Ampicillin Dry Syp- 8 145C 161 Each 5ml to contain: Ampicillin 125mg 30ml Bott 10959000 109590 65754 Cephalexin Syp- 9 145C 168 Each 5ml to contain: Cephalexin 125mg 30ml Bott 32874000 328740 197244 Erthyromycin Oral Susp- 10 145C 172 Each 5ml to contain: Erythromycin 125mg 30ml Bott 2400000 24000 14400 Amoxy & Clavulanic Acid Dry Syp- 11 145C 183 Each 5ml to contain: Amoxycillin 200 mg, Clavulanic Acid 28.5 mg 30ml Bott 35000000 350000 210000 Pyrazinamide Kid Tab/Cap- 12 145C 191 Each Kid Tab/Cap to contain: Pyrazinamide 300mg 1 Tab/Cap 500000 5000 3000 Chloroquine Syp- 13 145C 196 Each 5ml to contain: Chloroquine Phosphate 50mg. -

Modifications to the Harmonized Tariff Schedule of the United States To
U.S. International Trade Commission COMMISSIONERS Shara L. Aranoff, Chairman Daniel R. Pearson, Vice Chairman Deanna Tanner Okun Charlotte R. Lane Irving A. Williamson Dean A. Pinkert Address all communications to Secretary to the Commission United States International Trade Commission Washington, DC 20436 U.S. International Trade Commission Washington, DC 20436 www.usitc.gov Modifications to the Harmonized Tariff Schedule of the United States to Implement the Dominican Republic- Central America-United States Free Trade Agreement With Respect to Costa Rica Publication 4038 December 2008 (This page is intentionally blank) Pursuant to the letter of request from the United States Trade Representative of December 18, 2008, set forth in the Appendix hereto, and pursuant to section 1207(a) of the Omnibus Trade and Competitiveness Act, the Commission is publishing the following modifications to the Harmonized Tariff Schedule of the United States (HTS) to implement the Dominican Republic- Central America-United States Free Trade Agreement, as approved in the Dominican Republic-Central America- United States Free Trade Agreement Implementation Act, with respect to Costa Rica. (This page is intentionally blank) Annex I Effective with respect to goods that are entered, or withdrawn from warehouse for consumption, on or after January 1, 2009, the Harmonized Tariff Schedule of the United States (HTS) is modified as provided herein, with bracketed matter included to assist in the understanding of proclaimed modifications. The following supersedes matter now in the HTS. (1). General note 4 is modified as follows: (a). by deleting from subdivision (a) the following country from the enumeration of independent beneficiary developing countries: Costa Rica (b).