Sepsis-Induced Cholestasis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Denture-Associated Oral Microbiome and Periodontal Disease Causing
Case Report Gastroenterol Res. 2018;11(3):241-246 Denture-Associated Oral Microbiome and Periodontal Disease Causing an Anaerobic Pyogenic Liver Abscess in an Immunocompetent Patient: A Case report and Review of the Literature Muhammad Bader Hammamia, f, Elizabeth M. Noonanb, Anuj Chhapariac, Feras Al Khatibd, Juri Bassunere, Christine Hachema Abstract lowing peritonitis due to intra-abdominal bowel leakage with subsequent spread to the liver through the portal circulation [1] Pyogenic liver abscesses (PLA) develop from the spread of infection or via direct spread from biliary infections [2, 3]. They may through the portal circulation, biliary infections or arterial hematog- also result from arterial hematogenous seeding in the setting enous seeding in the setting of systemic infections. PLA are often of systemic infections, such as in cases of endocarditis or sep- poly-microbial and are uncommonly reported to be due to anaerobic tic thrombophlebitis [4]. PLA are often poly-microbial and are species. We report the case of a previously healthy, immunocompe- uncommonly reported to be due to anaerobic species [5, 6]. tent 63-year-old man with hepatic abscesses as a result of Fusobac- We report the case of an otherwise healthy immunocompetent terium nucleatum periodontal disease. In addition, a systemic review patient who developed multiple hepatic abscesses caused by of the literature is performed. Fusobacterium is a very rare cause of Fusobacterium nucleatum. Additionally, we review and sum- PLA in immunocompetent hosts with only a handful of cases reported marize the literature on PLA. in the literature. Although anaerobic infections such as Fusobacte- rium most often occur in immunocompromised individuals, clinicians Case Report should have a high index of suspicion in immunocompetent patients with periodontal disease or chronic stomatitis. -

Acalculous Cholecystitis Secondary to Giant Hepatic Abscess. Case Report and Literature Review
ARC Journal of Surgery Volume 6, Issue 1, 2020, PP 11-15 ISSN No. (Online) 2455-572X DOI: https://doi.org/10.20431/2455-572X.0601004 www.arcjournals.org Acalculous Cholecystitis Secondary to Giant Hepatic Abscess. Case Report and Literature Review Alberto Robles Méndez Hernández1*, Alejandro Vela Torres1, Yolik Ramírez-Marín2, Kelly Milla Hernández1, Roberto Jauregui Brechu1 1General Surgery Department, Hospital Angeles Metropolitano, Mexico City, Mexico 2General Surgery Department, Hospital General La Villa, Surgery Department, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City, Mexico *Corresponding Author: Alberto Robles Méndez Hernández, General Surgery Service, Hospital Ángeles Metropolitano, Tlacotalpan #59, Mexico City, Mexico. Abstract Alithiasic cholecystitis (AC) occurs in 5% of cases of acute cholecystitis, typically in severe patients, treatment of liver abscesses according to size is usually antibiotic therapy and radiological drainage, in refractory cases it may be consider surgical. Clinical case: A 75-year-old male patient with an 11-day history of nonspecific abdominal pain, evidenced by computed axial tomography anhepatic lesion of 134 mm diameter, was approached laparoscopically in which evidence of cholecystitis and liver abscess was evident and resolved. Results: The patient probably presented a simple hepatic cyst, a lesion from 10 previous years, that was infected with E, coli, with subsequent development of AC due to the infection. The resolution of the primary pathologyits complications by laparoscopic was feasible. Conclusions: The treatment of the primary cause and of the AC is indispensable for the clinical improvement of the patient, the laparoscopic treatment is considered as a safe option to approach the two entities with less morbidity than open surgery. -

Liver Abscess and Bacteremia Caused by Lactobacillus: Role of Probiotics
Sherid et al. BMC Gastroenterology (2016) 16:138 DOI 10.1186/s12876-016-0552-y CASE REPORT Open Access Liver abscess and bacteremia caused by lactobacillus: role of probiotics? Case report and review of the literature Muhammed Sherid1, Salih Samo2, Samian Sulaiman3, Husein Husein4, Humberto Sifuentes1 and Subbaramiah Sridhar1* Abstract Background: Lactobacilli are non-spore forming, lactic acid producing, gram-positive rods. They are a part of the normal gastrointestinal and genitourinary microbiota and have rarely been reported to be the cause of infections. Lactobacilli species are considered non-pathogenic organisms and have been used as probiotics to prevent antibiotic associated diarrhea. There are sporadic reported cases of infections related to lactobacilli containing probiotics. Case presentation: In this paper we discuss a case of an 82 year old female with liver abscess and bacteremia from lactobacillus after using probiotics containing lactobacilli in the course of her treatment of Clostridium difficile colitis. The Lactobacillus strain identification was not performed and therefore, both commensal microbiota and the probiotic product should be considered as possible sources of the strain. Conclusion: Lactobacilli can lead to bacteremia and liver abscesses in some susceptible persons and greater awareness of this potential side effect is warranted with the increasing use of probiotics containing lactobacilli. Keywords: Liver abscess, Lactobacillus, Probiotics, Cholecystectomy Background endocarditis cases), cancer (especially leukemia), total Lactobacilli are facultative anaerobic, non-spore form- parenteral nutrition use, broad spectrum antibiotic ing, lactic acid producing, and Gram positive bacilli. use, chronic kidney disease, inflammatory bowel dis- They are found in the normal microbiota of the oral ease, pancreatitis, chemotherapy, neutropenia, organ cavity, gastrointestinal tract, and female genitourinary transplantation (especially liver transplantation), HIV tract. -

An Atypical Presentation of Intrahepatic Perforated Cholecystitis: a Modern Indication to Open Cholecystectomy
Donati et al. BMC Surgery 2014, 14:6 http://www.biomedcentral.com/1471-2482/14/6 CASE REPORT Open Access An atypical presentation of intrahepatic perforated cholecystitis: a modern indication to open cholecystectomy. Report of a case Marcello Donati*, Antonio Biondi, Francesco Basile and Salvatore Gruttadauria Abstract Background: Intrahepatic gallbladder perforation with chronic liver abscess formation was anecdotically reported in the literature. The aim of this work is to report a case of intrahepatic gallbladder perforation and its atypical clinical presentation. Case presentation: A 62-year-old male patient came to our observation; his medical history showed intermittent fever up to 39-40°C of about 2 weeks and anorexia, with an overall weight loss of about 12 Kg. Physical examination of the abdomen was negative. An ultrasound of the liver and an abdominal CT angiogram detected a disomogeneous hypoechoic-hypodense area in the 5th segment of the liver. Differential diagnosis between hepatic abscess or gallbladder cancer remained open. A surgical exploration was planned. After laparoscopic exploration, a conversion to open procedure with an atypical resection of the 5th hepatic segment was performed. Histologic examination of the specimen showed an intrahepatic chronic perforation of the gallbladder with intrahepatic abscess. Conclusion: To the best of our knowledge, 18 cases have been reported in the literature as a Niemeier type I perforation. Clinical presentation, even in its extreme rarity, is more often acute. Differential diagnosis -

Hepatic Abscess Following NSAID Use in an Adolescentq
J Ped Surg Case Reports 2 (2014) 33e36 Contents lists available at ScienceDirect Journal of Pediatric Surgery CASE REPORTS journal homepage: www.jpscasereports.com Hepatic abscess following NSAID use in an adolescentq Margaret E. Clark a,*, Andrew W. Osten b, Mazen I. Abbas b, Mary J. Edwards a a Department of General Surgery, Tripler Army Medical Center, 1 Jarrett White Rd., Honolulu, HI 96859, USA b Department of Pediatrics, Tripler Army Medical Center, 1 Jarrett White Rd., Honolulu, HI 96859, USA article info abstract Article history: Non-steroidal anti-inflammatory drugs (NSAIDs) are a known cause of peptic ulcer disease, resulting in Received 3 December 2013 gastrointestinal bleeding or perforation. We present a case of a sixteen year old male athlete who pre- Received in revised form sented with abdominal pain and was found to have a pyogenic liver abscess secondary to a gastrohepatic 7 December 2013 fistula due to a deeply penetrating ulcer from NSAID use. This patient was successfully managed with Accepted 11 December 2013 antibiotics, a proton pump inhibitor (PPI), percutaneous drainage, and bowel rest. Perforating peptic ulcer disease (PPU) is rare in children, and this is a novel report of a resulting gastrohepatic fistula and subcapsular hepatic abscess. In otherwise healthy adolescents with abdominal complaints, a careful Key words: NSAID history of NSAID use should be obtained. Peptic ulcer Published by Elsevier Inc. Adolescent Gastrohepatic fistula Ulcer perforation Peptic ulcer disease (PUD) is a significant source of morbidity (CT) of the abdomen. He was hospitalized for pain control and and mortality in adults, but is rare in the pediatric population. -

Liver Abscess in the Tropics: Experience in the University Hospital, Kuala Lumpur
Postgrad Med J: first published as 10.1136/pgmj.63.741.551 on 1 July 1987. Downloaded from Postgraduate Medical Journal (1987) 63, 551-554 Tropical Medicine Liver abscess in the tropics: experience in the University Hospital, Kuala Lumpur. K.L. Goh, N.W. Wong, M. Paramsothy, M. Nojeg and K. Somasundaram Department ofMedicine, Faculty ofMedicine, University ofMalaya, 59100 Kuala Lumpur, Malaysia. Summary: We reviewed 204 cases ofliver abscess seen between 1970 and 1985. Ninety were found to be amoebic, 24 pyogenic and one tuberculous. The cause ofthe abscesses in the remaining 89 patients was not established. The patients were predominantly male, Indians, and in the 30-60 age group. The majority ofpatients presented with fever and right hypochondrial pain. The most common laboratory findings were leucocytosis, hypoalbnminaemia and an elevated serum alkaline phosphatase. Amoebic abscesses were mainly solitary while pyogenic abscesses were mainly multiple. Complications were few in our patients and included rupture into the pleural and peritoneal cavities and septicaemic shock. An overall mortality of 2.9% was recorded. The difficulty in diagnosing the abscess type is highlighted. The single most important test in helping us diagnose amoebic abscess, presumably the most common type of abscess in the tropics, is the Entamoeba histolytica antibody assay. This test should be used more frequently in the tropics. copyright. Introduction Liver abscess is a fairly common disease in Malaysia. autopsy, radionuclide scanning, ultrasonography or Balasegaram' reported 442 cases seen over a 15-year computed tomographic (CT) scanning. period. While it can be said that his experience is that Patients were considered to have an amoebic liver ofa referral surgical centre, many cases ofliver abscess abscess when (i) E. -

Hiccups : an Uncommon Presentation of Pyogenic Liver Abscess
92 LETTER Hiccups : an uncommon presentation of pyogenic liver abscess Z.F. Wu1, Y.C. Hsu2, W.C. Tseng2 (1) Department of Anesthesiology, Chi Mei Medical Center, Tainan, Taiwan, R.O.C. ; (2) Department of Anesthesiology, Tri-Service General Hospital and National Defense Medical Center, Taipei, Taiwan, R.O.C. To the Editor, Pyogenic liver abscess (PLA) is a rare but potentially life-threatening disease. It often presents with nonspecific symptoms and laboratory abnormalities (1, 2), which may result in missed diagnoses at emergency departments. Herein, we would like to report an uncommon presentation of PLA, which led to delayed diagnosis and interventions, in a patient with long-term malnutrition and relative immunocompromised status. An 81-year-old man presented to our emergency Figure 1. — Non-contrast CT of the abdomen revealed a department with persistent hiccups for 2 weeks and heterogeneous lesion with air bubbles in the liver (arrowheads) intermittent fever in recent 3 days. He had a history of in transverse view (A), which was close to right hemidiaphragm with development of reactive pleural effusion in coronal view pancreatic cancer experiencing pancreaticoduodenectomy (B). two years ago and long-term malnutrition. Except tachycardia (126 beats per minute) and hypotension (88/42 mm Hg), initial evaluations revealed no other tate aminotransferase level of 75 U/L. Additionally, obvious abnormalities. Laboratory abnormalities showed extremely high procalcitonin concentration of 82.79 an elevated creatinine level of 1.5 mg/dL and aspar- ng/mL implied a severe bacterial infection. Under the impression of septic shock, the patient received resuscitation and vasoactive treatment, and then was Table 1. -

Liver Abscess As a Rare Complication of Crohn's Disease: a Case Report Crohn Hastalığının Nadir Bir Komplikasyonu Olarak Karaciğer Absesi: Olgu Sunumu
Turk J Gastroenterol 2004; 15 (1): 45-48 Liver abscess as a rare complication of Crohn's disease: A case report Crohn hastalığının nadir bir komplikasyonu olarak karaciğer absesi: Olgu sunumu Çetin KARACA, Binnur PINARBAŞI, Ahmet DANALIOĞLU, Filiz AKYÜZ, Sabahattin KAYMAKOĞLU, Sadakat ÖZDİL, Güngör BOZTAŞ, Zeynel MUNGAN İstanbul University, istanbul Medical Faculty, Gastroenterohepatology Department, İstanbul Pyogenic liver abscess is a rarely seen extraintestinal complica- Piyojenik karaciğer absesi Crohn hastalığının nadir görülen bir tion of Crohn's disease. It has different features from other liver ekstraintestinal komplikasyonu olup, diğer karaciğer abselerin- abscesses. Its clinical and laboratory findings are not specific den farklı özellikleri mevcuttur. Klinik ve laboratuar bulguları and mimic the reactivation of Crohn's disease and diagnosis nonspesifiktir. Crohn hastalığının reaktivasyonu ile karışabi- can be delayed. The radiological methods are very useful in di- lir ve tanı konulması da bu nedenle gecikebilir. agnosis and treatment of liver abscess. In this paper, we present Karaciğer absesinin tanı ve tedavisinde radyolojik metodlar ol- a patient with pyogenic liver abscess which developed in the co- dukça yararlıdır. Bu yazıda, Crohn hastalığı seyrinde piyojenik urse of Crohn's disease. karaciğer hastalığı gelişen bir hasta sunulmuştur. Key words: Liver abscess, Crohn's disease Anahtar kelimeler: Karaciğer absesi, Crohn hastalığı INTRODUCTION Crohn's disease (CD) is a chronic inflammatory di- 100,000 and it is more often observed in younger sease that can involve all portions of the gastroin- ages (2). Sometimes LA is misdiagnosed as reacti- testinal tract from mouth to anus. The gross mu- vation of CD because of shared symptoms and fin- cosal lesions typically begin as aphthous ulcers. -

A Multiple Choice Answer?
CASE REPORTS A Multiple Choice Answer? Christina To, MD David Geffen School of Medicine at UCLA, Los Angeles, California. Jason Napolitano, MD Disclosure: None of the authors have any conflicts of interest associated with the work presented in this manuscript. Authorship: All authors had access to the data and played a role in writing this manuscript. Originality: The work represented in this manuscript is original, and the submission is not under review by any other publication. KEYWORDS: abscess, fusobacterium, liver, pyogenic. A 49-year-old man with a history of hypertension presented With continued antibiotic treatment, the patient’s fevers to our hospital with a 2-week history of sharp pain in the resolved and leukocytosis improved. A follow-up CT abdo- right upper abdomen and right lower chest radiating to the men/pelvis obtained on hospital day 10 showed a reduction back. The patient reported a few days of fevers, chills, in size of the multiple liver abscesses. There was also drenching night sweats, shortness of breath, malaise, and increased prominence of the appendix with mild stranding. fatigue. He denied recent travel. Vital signs were tempera- The patient was taken for appendectomy. Pathology was ture 38.4C, blood pressure 119/74 mmHg, heart rate consistent with acute appendicitis with focal fat necrosis. 95 beats/minutes, respiratory rate 16 breaths/minutes, and The patient was ultimately discharged with the plan being oxygen saturation 96% on 5 L nasal cannula. Physical exam- to continue ertapenem until radiographic resolution of all ination revealed poor dentition, right upper abdominal the abscesses was demonstrated. -
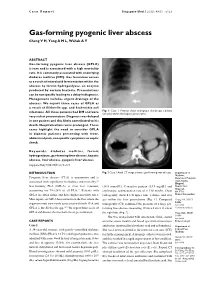
Gas-Forming Pyogenic Liver Abscess Chong V H, Yong a M L, Wahab a Y
Case Report Singapore Med J 2008; 49(5) : e123 Gas-forming pyogenic liver abscess Chong V H, Yong A M L, Wahab A Y ABSTRACT Gas-forming pyogenic liver abscess (GPLA) is rare and is associated with a high mortality rate. It is commonly associated with underlying diabetes mellitus (DM). Gas formation occurs as a result of mixed acid fermentation within the abscess by formic hydrogenlyase, an enzyme produced by certain bacteria. Presentations can be nonspecific leading to a delay in diagnosis. Management includes urgent drainage of the abscess. We report three cases of GPLA as a result of Klebsiella spp. and Escherichia coli infections. All three patients had DM and were Fig. 1 Case 1. Frontal chest radiograph shows gas pockets (arrows) within the hepatic parenchyma. very sick at presentation. Diagnosis was delayed in one patient and this likely contributed to his death. Hospitalisations were prolonged. These cases highlight the need to consider GPLA in diabetic patients presenting with fever, abdominal pain, nonspecific symptoms or septic shock. Keywords: diabetes mellitus, formic hydrogenlyase, gas-forming liver abscess, hepatic abscess, liver abscess, pyogenic liver abscess Singapore Med J 2008; 49(5): e123-e125 INTRODUCTION Fig. 2 Case 1. Axial CT image shows a gas-forming liver abscess. Department of Medicine, Pyogenic liver abscess (PLA) is uncommon and is Raja Isteri Pengiran (1) Anak Saleha associated with significant morbidities and mortality. Hospital, Gas-forming PLA (GPLA) is even less common, (18.8 mmol/L), C-reactive protein (33.9 mg/dL) and Bandar Seri Begawan, (2) accounting for 7%–24% of all PLA. -

Laparoscopic Surgery for Intra-Abdominal Ruptured Liver Abscess: a Study of 32 Cases
Research Article Open Access J Surg Volume 10 Issue 5 - June 2019 Copyright © All rights are reserved by Huynh Quang Huy DOI: 10.19080/OAJS.2019.10.555798 Laparoscopic Surgery for Intra-abdominal Ruptured Liver Abscess: A study of 32 cases Pham Hong Duc1*, Le Quang Minh2, Le Cong Tri3, Vu Thi Minh Thuc4, Huynh Quang Huy5, Ho Hoang Phuong6 and Nguyen Quoc Vinh7 1Radiology Department, Ha Noi Medical University, Vietnam 2Department of Health, Ministry of Public Security, Vietnam 3Surgery Department, Cho Ray hospital, Vietnam 4National Otorhinolaryngology Hospital of Vietnam 5Radiology Department, Pham Ngoc Thach University of Medicine and HCMC Oncology Hospital, Vietnam 6Radiology Department, HCMC Medical and Pharmacy University 7General Surgery Department, HCMC Medical and Pharmacy University Hospital Received: May 27, 2019; Published: June 25, 2019 *Corresponding author: Pham Hong Duc, Radiology Department, Ha Noi Medical University, Vietnam Abstract Objectives: liver abscess. The purpose of this study is to see the efficacy of laparoscopic treatment in the management of intra-abdominal ruptured Patients and Methods: postoperative hospital stay was From retrospectively 2014 to 2018, analyzed. 32 patients with intra-abdominal ruptured liver abscess meeting entry criteria received laparoscopic surgical management in our hospital. Clinical data including operation time, postoperative complication rate, length of Results: 32 patients with a median age of 53.3 ± 15.3 years (range, 24–85 years). The mean of operating time was 105 ± 28 minutes (median 100 minutes, range: 60 - 185 minutes). Time to pass gas after surgery was 2,8 ± 1,6 days in average (range: 1 - 6 days). The mean of time to remove drains was 10 ± 5 days (range: 3 - 27 days). -

Cryptogenic Pyogenic Liver Abscess Due to Fusobacterium Nucleatum: a Case Report
Ryan Wilson, BSc, Ryan LeBlanc, BScPharm, ACPR, Abu A. Hamour, MBBS, MSc, FRCP(Edin), FRCP Cryptogenic pyogenic liver abscess due to Fusobacterium nucleatum: A case report Clinicians should consider the possibility of pyogenic liver abscess when patients present with fever, right upper quadrant pain, and shortness of breath. ABSTRACT: Pyogenic liver abscess- ormation of a pyogenic liver one case series identifying anaerobes es are relatively uncommon but po- abscess (PLA) is potentially in up to 45% of cases.11 Typically, sys- tentially life-threatening. Most in- F life-threatening, with an esti- temic F. nucleatum infections occur in fections that lead to an abscess are mat ed incidence rate of 2.3 cases immunocompromised individuals or associated with underlying biliary per 100 000 patients.1,2 Despite recent are of odontogenic origin.7-9,12 disease, or are due to hematogenous advances in the investigation and spread from a variety of nonbiliary management of such abscesses, the Case data sites. Although most infections are mortality rate still ranges from 2% to A 44-year-old woman presented to the polymicrobial, monomicrobial Fuso- 12%.3,4 Amebic liver abscess is the emergency room with a 6-day history bacterium nucleatum abscesses do most common form of liver abscess of fever, chills, rigor, vomiting, head- occur. These exceedingly rare mono- worldwide, whereas PLA is the most ache, and shortness of breath. She microbial infections typically occur common form in North America,5 had diffuse abdominal pain that had in immunocompromised individuals and is usually the result of polymi- become localized to the right upper or in the presence of periodontal crobial infection.6 It is believed that quadrant, and had progressed to being disease.