Volume 7 Number 7
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(Crone Et Al.) S1. List of Studies with Movement In
Supplementary material: Mixed use landscapes can promote range expansion (Crone et al.) S1. List of studies with movement in high- and low-quality environments 1 Allema, B., van der Werf, W., van Lenteren, J. C., Hemerik, L. & Rossing, W. A. H. Movement behaviour of the carabid beetle Pterostichus melanarius in crops and at a habitat interface explains patterns of population redistribution in the field. PLoS One 9 (2014). 2 Avgar, T., Mosser, A., Brown, G. S. & Fryxell, J. M. Environmental and individual drivers of animal movement patterns across a wide geographical gradient. J. Anim. Ecol. 82, 96-106 (2013). 3 Brouwers, N. C. & Newton, A. C. Movement analyses of wood cricket (Nemobius sylvestris) (Orthoptera: Gryllidae). Bulletin of Entomological Research 100, 623-634 (2010). 4 Brown, L. M. et al. Using animal movement behavior to categorize land cover and predict consequences for connectivity and patch residence times. Landscape Ecol 32, 1657-1670 (2017). 5 Capinera, J. L. & Barbosa, P. Dispersal of first-instar gypsy moth larvae in relation to population quality. Oecologia 26, 53-64 (1976). 6 Cartar, R. V. & Real, L. A. Habitat structure and animal movement: the behaviour of bumble bees in uniform and random spatial resource distributions. Oecologia 112, 430- 434 (1997). 7 Chapman, D. S., Dytham, C. & Oxford, G. S. Landscape and fine-scale movements of a leaf beetle: the importance of boundary behaviour. Oecologia 154, 55-64 (2007). 8 Claussen, D. L., Finkler, M. S. & Smith, M. M. Thread trailing of turtles: methods for evaluating spatial movements and pathway structure. Canadian Journal of Zoology 75, 2120-2128 (1997). -

Phylogenetic Relationships of Phyciodes Butterfly Species (Lepidoptera: Nymphalidae): Complex Mtdna Variation and Species Delimitations
Systematic Entomology (2003) 28, 257±273 Phylogenetic relationships of Phyciodes butterfly species (Lepidoptera: Nymphalidae): complex mtDNA variation and species delimitations NIKLAS WAHLBERG*, RITA OLIVEIRA* andJAMES A. SCOTTy *Metapopulation Research Group, Department of Ecology and Systematics, University of Helsinki, Finland, and yLakewood, Colorado, U.S.A. Abstract. Mitochondrial DNA variation wasstudied in the butterfly genus Phyciodes (Lepidoptera: Nymphalidae) by sequencing 1450 bp of the COI gene from 140 individuals of all eleven currently recognized species. The study focused on four species in particular that have been taxonomically difficult for the past century, P. tharos, P. cocyta, P. batesii and P. pulchella. A cladistic analysis of ninety-eight unique haplotypesshowedthat Phyciodes formsa monophyletic group with P. graphica as the most basal species. Of the three informal species groupsdescribed for Phyciodes, only one (the mylitta-group) isunambiguously monophyletic. Within the tharos-group, seven well supported clades were found that correspond to three taxa, P. tharos, P. pulchella and a grade consisting of P. cocyta and P. batesii haplotypesinterdigitated with each other. None of the clades is formed exclusively by one species. The patterns of haplotype variation are the result of both retained ancient polymorphism and introgression. Introgression appearsto be mostcommon between P. cocyta and P. batesii; however, these two species occur sympatrically and are morphologically and ecologically distinct, suggesting that the level of current introgression does not seem to be enough to threaten their genetic integrity. The results indicate that mitochondrial DNA sequences must be used with great caution in delimiting species, especially when infraspecific samples are few, or introgression seems to be rampant. -

Butterflies of the Wesleyan Campus
BUTTERFLIES OF THE WESLEYAN CAMPUS SWALLOWTAILS Hairstreaks (Subfamily - Theclinae) (Family PAPILIONIDAE) Great Purple Hairstreak - Atlides halesus Coral Hairstreak - Satyrium titus True Swallowtails Banded Hairstreak - Satyrium calanus (Subfamily - Papilioninae) Striped Hairstreak - Satyrium liparops Pipevine Swallowtail - Battus philenor Henry’s Elfin - Callophrys henrici Zebra Swallowtail - Eurytides marcellus Eastern Pine Elfin - Callophrys niphon Black Swallowtail - Papilio polyxenes Juniper Hairstreak - Callophrys gryneus Giant Swallowtail - Papilio cresphontes White M Hairstreak - Parrhasius m-album Eastern Tiger Swallowtail - Papilio glaucus Gray Hairstreak - Strymon melinus Spicebush Swallowtail - Papilio troilus Red-banded Hairstreak - Calycopis cecrops Palamedes Swallowtail - Papilio palamedes Blues (Subfamily - Polommatinae) Ceraunus Blue - Hemiargus ceraunus Eastern-Tailed Blue - Everes comyntas WHITES AND SULPHURS Spring Azure - Celastrina ladon (Family PIERIDAE) Whites (Subfamily - Pierinae) BRUSHFOOTS Cabbage White - Pieris rapae (Family NYMPHALIDAE) Falcate Orangetip - Anthocharis midea Snouts (Subfamily - Libytheinae) American Snout - Libytheana carinenta Sulphurs and Yellows (Subfamily - Coliadinae) Clouded Sulphur - Colias philodice Heliconians and Fritillaries Orange Sulphur - Colias eurytheme (Subfamily - Heliconiinae) Southern Dogface - Colias cesonia Gulf Fritillary - Agraulis vanillae Cloudless Sulphur - Phoebis sennae Zebra Heliconian - Heliconius charithonia Barred Yellow - Eurema daira Variegated Fritillary -

Lepidoptera of North America 5
Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera by Valerio Albu, 1411 E. Sweetbriar Drive Fresno, CA 93720 and Eric Metzler, 1241 Kildale Square North Columbus, OH 43229 April 30, 2004 Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Cover illustration: Blueberry Sphinx (Paonias astylus (Drury)], an eastern endemic. Photo by Valeriu Albu. ISBN 1084-8819 This publication and others in the series may be ordered from the C.P. Gillette Museum of Arthropod Diversity, Department of Bioagricultural Sciences and Pest Management Colorado State University, Fort Collins, CO 80523 Abstract A list of 1531 species ofLepidoptera is presented, collected over 15 years (1988 to 2002), in eleven southern West Virginia counties. A variety of collecting methods was used, including netting, light attracting, light trapping and pheromone trapping. The specimens were identified by the currently available pictorial sources and determination keys. Many were also sent to specialists for confirmation or identification. The majority of the data was from Kanawha County, reflecting the area of more intensive sampling effort by the senior author. This imbalance of data between Kanawha County and other counties should even out with further sampling of the area. Key Words: Appalachian Mountains, -

Impact of Non-Lethal Genetic Sampling on the Survival, Longevity and Behaviour of the Hermes Copper (Lycaena Hermes) Butterfly
Insect Conservation and Diversity (2013) doi: 10.1111/icad.12024 Impact of non-lethal genetic sampling on the survival, longevity and behaviour of the Hermes copper (Lycaena hermes) butterfly 1 2 3 DANIEL A. MARSCHALEK, JULIA A. JESU and MARK E. BERRES 1Department of Entomology, University of Wisconsin, Madison, WI, USA, 2Biology Department, San Diego State University, San Diego, CA, USA and 3Department of Animal Sciences, University of Wisconsin, Madison, WI, USA Abstract. 1. Genetic techniques are important tools for conservation, but tissue sampling for DNA analysis can be particularly detrimental to small study organisms. Historically, obtaining DNA samples from small insects and butter- flies has involved destructive (lethal) methods. 2. Recent improvements to DNA purification technologies have increased the likelihood that non-lethal sampling will be successful. In spite of this, only a few studies have evaluated the impacts of sampling on survival and behaviour. 3. The Hermes copper, Lycaena hermes (Edwards), butterfly has a restricted distribution and generally less than 10 individuals are encountered at any one location. Non-lethal DNA sampling would allow for genetic studies that have the potential to augment conservation decisions without causing local extirpations. 4. We demonstrate that removing a leg from an adult male Hermes copper does not have a measureable effect on their survival, longevity or behaviour. In addition, a single leg provides a sufficient DNA sample for amplified fragment length polymorphism studies. 5. The Hermes copper butterfly represents the smallest butterfly species for which the survival and behaviour has been assessed in relation to non-lethal tis- sue sampling. This suggests that research involving smaller and more delicate species could utilise leg removal as a non-lethal genetic sampling technique. -

Orange Sulphur, Colias Eurytheme, on Boneset
Orange Sulphur, Colias eurytheme, on Boneset, Eupatorium perfoliatum, In OMC flitrh Insect Survey of Waukegan Dunes, Summer 2002 Including Butterflies, Dragonflies & Beetles Prepared for the Waukegan Harbor Citizens' Advisory Group Jean B . Schreiber (Susie), Chair Principal Investigator : John A. Wagner, Ph . D . Associate, Department of Zoology - Insects Field Museum of Natural History 1400 South Lake Shore Drive Chicago, Illinois 60605 Telephone (708) 485 7358 home (312) 665 7016 museum Email jwdw440(q-), m indsprinq .co m > home wagner@,fmnh .orq> museum Abstract: From May 10, 2002 through September 13, 2002, eight field trips were made to the Harbor at Waukegan, Illinois to survey the beach - dunes and swales for Odonata [dragonfly], Lepidoptera [butterfly] and Coleoptera [beetles] faunas between Midwest Generation Plant on the North and the Outboard Marine Corporation ditch at the South . Eight species of Dragonflies, fourteen species of Butterflies, and eighteen species of beetles are identified . No threatened or endangered species were found in this survey during twenty-four hours of field observations . The area is undoubtedly home to many more species than those listed in this report. Of note, the endangered Karner Blue butterfly, Lycaeides melissa samuelis Nabakov was not seen even though it has been reported from Illinois Beach State Park, Lake County . The larval food plant, Lupinus perennis, for the blue was not observed at Waukegan. The limestone seeps habitat of the endangered Hines Emerald dragonfly, Somatochlora hineana, is not part of the ecology here . One surprise is the. breeding population of Buckeye butterflies, Junonia coenid (Hubner) which may be feeding on Purple Loosestrife . The specimens collected in this study are deposited in the insect collection at the Field Museum . -

Phylogenetic Relationships and Historical Biogeography of Tribes and Genera in the Subfamily Nymphalinae (Lepidoptera: Nymphalidae)
Blackwell Science, LtdOxford, UKBIJBiological Journal of the Linnean Society 0024-4066The Linnean Society of London, 2005? 2005 862 227251 Original Article PHYLOGENY OF NYMPHALINAE N. WAHLBERG ET AL Biological Journal of the Linnean Society, 2005, 86, 227–251. With 5 figures . Phylogenetic relationships and historical biogeography of tribes and genera in the subfamily Nymphalinae (Lepidoptera: Nymphalidae) NIKLAS WAHLBERG1*, ANDREW V. Z. BROWER2 and SÖREN NYLIN1 1Department of Zoology, Stockholm University, S-106 91 Stockholm, Sweden 2Department of Zoology, Oregon State University, Corvallis, Oregon 97331–2907, USA Received 10 January 2004; accepted for publication 12 November 2004 We infer for the first time the phylogenetic relationships of genera and tribes in the ecologically and evolutionarily well-studied subfamily Nymphalinae using DNA sequence data from three genes: 1450 bp of cytochrome oxidase subunit I (COI) (in the mitochondrial genome), 1077 bp of elongation factor 1-alpha (EF1-a) and 400–403 bp of wing- less (both in the nuclear genome). We explore the influence of each gene region on the support given to each node of the most parsimonious tree derived from a combined analysis of all three genes using Partitioned Bremer Support. We also explore the influence of assuming equal weights for all characters in the combined analysis by investigating the stability of clades to different transition/transversion weighting schemes. We find many strongly supported and stable clades in the Nymphalinae. We are also able to identify ‘rogue’ -

Butterflies of the Finger Lakes National Forest
The Finger Lakes National Butterflies of "... the first precaution Forest comprises 16,000 acres of the of intelligent tinkering federally owned land between Cayuga and Seneca Lakes in Finger Lakes is to keep every cog and New York State. The Forest is wheel ... " managed for multiple uses to ~~Mut provide a variety of goods and ~Aldo Leopold services to a diverse public. Different programs include a This list compiled through the work of livestock grazing program, a Charles R. Smith, Ph.D. Department of Natural Resources small scale timber management Cornell University program, diverse habitats for And Donald Bright-Smith, Ph.D. wildlife and fish, and Department of Pathobiology recreational opportunities for TexasA&M multiple user groups. And made possible through the generosity New York State Chapter of the For more information about the Finger Lakes Wild Turkey Federation National Forest contact: Hector Ranger District 5218 State Route 414 United States Forest Eastern ~~1~ .. ~ Department of Service Region New York State Chapter Hector, NY 14841 Agriculture NWTF (607) 546-4470 Family Papilionidae: Swallowtails Family Hesperiidae: Skippers 0 Papilio polyxenes, Black Swallowtail 0 Epargyreus clarus, Silver-spotted 0 Pap ilia cresphontes, Giant Swallowtail Skipper 0 Papilio glaucus, Eastern Tiger Family Nymphalidae: Brushfoots 0 Erynnis ice/us, Dreamy Duskywing Swallowtail 0 Speyeria cybele, Great Spangled Fritillary D Erynnisjuvenalis, Juvenal's Duskywing 0 Pap ilia troilus, Spicebush Swallowtail 0 Boloria selene, Silver-bordered Fritillary -

2017, Jones Road, Near Blackhawk, RAIN (Photo: Michael Dawber)
Edited and Compiled by Rick Cavasin and Jessica E. Linton Toronto Entomologists’ Association Occasional Publication # 48-2018 European Skippers mudpuddling, July 6, 2017, Jones Road, near Blackhawk, RAIN (Photo: Michael Dawber) Dusted Skipper, April 20, 2017, Ipperwash Beach, LAMB American Snout, August 6, 2017, (Photo: Bob Yukich) Dunes Beach, PRIN (Photo: David Kaposi) ISBN: 978-0-921631-53-7 Ontario Lepidoptera 2017 Edited and Compiled by Rick Cavasin and Jessica E. Linton April 2018 Published by the Toronto Entomologists’ Association Toronto, Ontario Production by Jessica Linton TORONTO ENTOMOLOGISTS’ ASSOCIATION Board of Directors: (TEA) Antonia Guidotti: R.O.M. Representative Programs Coordinator The TEA is a non-profit educational and scientific Carolyn King: O.N. Representative organization formed to promote interest in insects, to Publicity Coordinator encourage cooperation among amateur and professional Steve LaForest: Field Trips Coordinator entomologists, to educate and inform non-entomologists about insects, entomology and related fields, to aid in the ONTARIO LEPIDOPTERA preservation of insects and their habitats and to issue Published annually by the Toronto Entomologists’ publications in support of these objectives. Association. The TEA is a registered charity (#1069095-21); all Ontario Lepidoptera 2017 donations are tax creditable. Publication date: April 2018 ISBN: 978-0-921631-53-7 Membership Information: Copyright © TEA for Authors All rights reserved. No part of this publication may be Annual dues: reproduced or used without written permission. Individual-$30 Student-free (Association finances permitting – Information on submitting records, notes and articles to beyond that, a charge of $20 will apply) Ontario Lepidoptera can be obtained by contacting: Family-$35 Jessica E. -

Heliantheae of Iowa. I
Proceedings of the Iowa Academy of Science Volume 36 Annual Issue Article 27 1929 Heliantheae of Iowa. I M. Rae Johns Let us know how access to this document benefits ouy Copyright ©1929 Iowa Academy of Science, Inc. Follow this and additional works at: https://scholarworks.uni.edu/pias Recommended Citation Johns, M. Rae (1929) "Heliantheae of Iowa. I," Proceedings of the Iowa Academy of Science, 36(1), 147-184. Available at: https://scholarworks.uni.edu/pias/vol36/iss1/27 This Research is brought to you for free and open access by the Iowa Academy of Science at UNI ScholarWorks. It has been accepted for inclusion in Proceedings of the Iowa Academy of Science by an authorized editor of UNI ScholarWorks. For more information, please contact [email protected]. Johns: Heliantheae of Iowa. I HELIANTHEAE OF row A. I lVI. RAE JOIINS Heliantheae is one of the nine tribes of the Family Compositae. It is believed to have originated in South America soon after the appearance of the Family and may be regarded as the oldest and most primitive of the tribes. The near approach to the earliest composite form is said to be retained in the more foliaceous outer invol ucral bracts, the more normally developed and more firmly attached paleae subtending the disk flowers, the 0 less transformed calyx-limb of persistent teeth or aristae directly continuous with the ribs of the ovary, and in some genera, the less firmly united or nearly free anthers. (Bentham, 1873.) The characters which today distinguish it from the other tribes are: Receptacle: chaffy, depressed, horizontal, convex, or columnar. -

List of Insect Species Which May Be Tallgrass Prairie Specialists
Conservation Biology Research Grants Program Division of Ecological Services © Minnesota Department of Natural Resources List of Insect Species which May Be Tallgrass Prairie Specialists Final Report to the USFWS Cooperating Agencies July 1, 1996 Catherine Reed Entomology Department 219 Hodson Hall University of Minnesota St. Paul MN 55108 phone 612-624-3423 e-mail [email protected] This study was funded in part by a grant from the USFWS and Cooperating Agencies. Table of Contents Summary.................................................................................................. 2 Introduction...............................................................................................2 Methods.....................................................................................................3 Results.....................................................................................................4 Discussion and Evaluation................................................................................................26 Recommendations....................................................................................29 References..............................................................................................33 Summary Approximately 728 insect and allied species and subspecies were considered to be possible prairie specialists based on any of the following criteria: defined as prairie specialists by authorities; required prairie plant species or genera as their adult or larval food; were obligate predators, parasites -
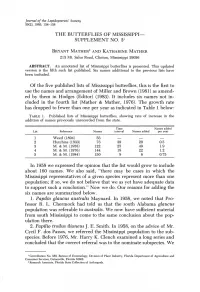
The Butterflies of Mississippi Supplement No
Journal of the Lepidopterists' Society 39(2), 1985, 134-138 THE BUTTERFLIES OF MISSISSIPPI SUPPLEMENT NO. 31 BRYANT MATHER2 AND KATHARINE MATHER 213 Mt. Salus Road, Clinton, Mississippi 39056 ABSTRACT. An annotated list of Mississippi butterflies is presented. This updated version is the fifth such list published. Six names additional to the previous lists have been included. Of the five published lists of Mississippi butterflies, this is the first to use the names and arrangement of Miller and Brown (1981) as amend ed by them in Hodges (Editor) (1983). It includes six names not in cluded in the fourth list (Mather & Mather, 1976). The growth rate has dropped to fewer than one per year as indicated in Table 1 below: TABLE 1. Published lists of Mississippi butterflies, showing rate of increase in the addition of names previously unrecorded from the state. Time Names added List Reference Names interval Names added per year 1 Weed (1894) 53 2 Hutchins (1933) 73 39 20 0.5 3 M. & M. (1958) 122 25 49 1.9 4 M. & M. (1976) 144 18 22 1.2 5 M. & M. (1984) 150 8 6 0.75 In 1958 we expressed the opinion that the list would grow to include about 160 names. We also said, "there may be cases in which the Mississippi representatives of a given species represent more than one population; if so, we do not believe that we as yet have adequate data to support such a conclusion." Now we do. Our reasons for adding the six names are summarized below. 1.