19910001805.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Meteor Activity Outlook for April 10-16, 2021
Meteor Activity Outlook for April 10-16, 2021 This brilliant fireball was captured by Uli Fehr on 10 March 2021, at 01:57 WET (1:57 UT) from Santa Cruz de Tenerife, Canary Islands, Spain. ©Uli Fehr www.fehrpics.com Note that this image is not intended to be used for accurate photometric assessment of meteor brightness. During this period the moon reaches its new phase on Sunday April 11th. On this date the moon is located near the sun and is invisible at night. As the week progresses the waxing crescent moon will enter the evening sky but will not cause any interference to meteor observers, especially during the more active morning hours. The estimated total hourly meteor rates for evening observers this week is near 2 as seen from mid-northern latitudes and 3 as seen from tropical southern locations (25S). For morning observers, the estimated total hourly rates should be near 7 as seen from mid- northern latitudes (45N) and 10 as seen from tropical southern locations (25S). The actual rates will also depend on factors such as personal light and motion perception, local weather conditions, alertness, and experience in watching meteor activity. Note that the hourly rates listed below are estimates as viewed from dark sky sites away from urban light sources. Observers viewing from urban areas will see less activity as only the brighter meteors will be visible from such locations. The radiant (the area of the sky where meteors appear to shoot from) positions and rates listed below are exact for Saturday night/Sunday morning April 10/11. -

Sky Notes by Neil Bone 2006 April & May
Sky notes by Neil Bone 2006 April & May New Moon falls on April 27 and May Sun and Moon 27, putting the darkest night-time skies in The planets the opening and closing weeks in this inter- Spring’s lengthening days, with the Sun val. The ecliptic plane – close to which the Mercury is very poorly placed in the climbing to a higher northerly declination on Moon’s orbit lies – still cuts a fairly steep morning sky during early April, rising the ecliptic (its apparent annual path against angle with the western horizon in early barely 30 minutes before the Sun at UK the star background), bring extended oppor- evening at this time of year, making early latitudes. Superior conjunction, on the tunities for solar observing. Using the safe April and early May favourable times to Sun’s far side, is reached on May 18, and projection method, observers can follow the observe the young waxing crescent Moon. by the end of the month, Mercury can be comings and goings of sunspots and the as- In the interval from 3−5 days after New, glimpsed as a magnitude –1 ‘spark’ low in sociated bright patches of faculae (clouds of the day-night line of the terminator cuts the northwestern sky about an hour after hot hydrogen overlying spot-forming re- across some of the Moon’s most interest- sunset. Viewing conditions will be much gions, usually best seen near the solar limb). ing terrain, which is thrown into relief by more favourable in mid-June. At the moment, however, sunspot activity the interplay of light and shadow under low Venus is a ‘Morning Star’, but at a decli- in the current 11-year cycle (number 23) is local solar illumination. -

Patrick Moore's Practical Astronomy Series
Patrick Moore’s Practical Astronomy Series Other Titles in this Series Navigating the Night Sky Astronomy of the Milky Way How to Identify the Stars and The Observer’s Guide to the Constellations Southern/Northern Sky Parts 1 and 2 Guilherme de Almeida hardcover set Observing and Measuring Visual Mike Inglis Double Stars Astronomy of the Milky Way Bob Argyle (Ed.) Part 1: Observer’s Guide to the Observing Meteors, Comets, Supernovae Northern Sky and other transient Phenomena Mike Inglis Neil Bone Astronomy of the Milky Way Human Vision and The Night Sky Part 2: Observer’s Guide to the How to Improve Your Observing Skills Southern Sky Michael P. Borgia Mike Inglis How to Photograph the Moon and Planets Observing Comets with Your Digital Camera Nick James and Gerald North Tony Buick Telescopes and Techniques Practical Astrophotography An Introduction to Practical Astronomy Jeffrey R. Charles Chris Kitchin Pattern Asterisms Seeing Stars A New Way to Chart the Stars The Night Sky Through Small Telescopes John Chiravalle Chris Kitchin and Robert W. Forrest Deep Sky Observing Photo-guide to the Constellations The Astronomical Tourist A Self-Teaching Guide to Finding Your Steve R. Coe Way Around the Heavens Chris Kitchin Visual Astronomy in the Suburbs A Guide to Spectacular Viewing Solar Observing Techniques Antony Cooke Chris Kitchin Visual Astronomy Under Dark Skies How to Observe the Sun Safely A New Approach to Observing Deep Space Lee Macdonald Antony Cooke The Sun in Eclipse Real Astronomy with Small Telescopes Sir Patrick Moore and Michael Maunder Step-by-Step Activities for Discovery Transit Michael K. -
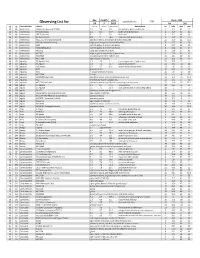
Observing List
day month year Epoch 2000 local clock time: 2.00 Observing List for 24 7 2019 RA DEC alt az Constellation object mag A mag B Separation description hr min deg min 39 64 Andromeda Gamma Andromedae (*266) 2.3 5.5 9.8 yellow & blue green double star 2 3.9 42 19 51 85 Andromeda Pi Andromedae 4.4 8.6 35.9 bright white & faint blue 0 36.9 33 43 51 66 Andromeda STF 79 (Struve) 6 7 7.8 bluish pair 1 0.1 44 42 36 67 Andromeda 59 Andromedae 6.5 7 16.6 neat pair, both greenish blue 2 10.9 39 2 67 77 Andromeda NGC 7662 (The Blue Snowball) planetary nebula, fairly bright & slightly elongated 23 25.9 42 32.1 53 73 Andromeda M31 (Andromeda Galaxy) large sprial arm galaxy like the Milky Way 0 42.7 41 16 53 74 Andromeda M32 satellite galaxy of Andromeda Galaxy 0 42.7 40 52 53 72 Andromeda M110 (NGC205) satellite galaxy of Andromeda Galaxy 0 40.4 41 41 38 70 Andromeda NGC752 large open cluster of 60 stars 1 57.8 37 41 36 62 Andromeda NGC891 edge on galaxy, needle-like in appearance 2 22.6 42 21 67 81 Andromeda NGC7640 elongated galaxy with mottled halo 23 22.1 40 51 66 60 Andromeda NGC7686 open cluster of 20 stars 23 30.2 49 8 46 155 Aquarius 55 Aquarii, Zeta 4.3 4.5 2.1 close, elegant pair of yellow stars 22 28.8 0 -1 29 147 Aquarius 94 Aquarii 5.3 7.3 12.7 pale rose & emerald 23 19.1 -13 28 21 143 Aquarius 107 Aquarii 5.7 6.7 6.6 yellow-white & bluish-white 23 46 -18 41 36 188 Aquarius M72 globular cluster 20 53.5 -12 32 36 187 Aquarius M73 Y-shaped asterism of 4 stars 20 59 -12 38 33 145 Aquarius NGC7606 Galaxy 23 19.1 -8 29 37 185 Aquarius NGC7009 -
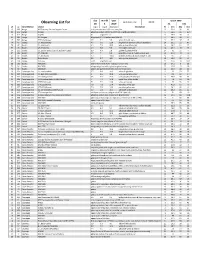
Observing List
day month year Epoch 2000 local clock time: 22.00 Observing List for 18 5 2019 RA DEC alt az Constellation object mag A mag B Separation description hr min deg min 10 305 Auriga M37 January Salt and Pepper Cluster rich, open cluster of 150 stars, very fine 5 52.4 32 33 20 315 Auriga IC2149 planetary nebula, stellar to small disk, slightly elongated 5 56.5 46 6.3 15 319 Auriga Capella 0.1 alignment star 5 16.6 46 0 21 303 Auriga UU Aurigae Magnitude 5.1-7.0 carbon star (AL# 36) 6 36.5 38 27 15 307 Auriga 37 Theta Aurigae 2.6 7.1 3.6 white & bluish stars 5 59.7 37 13 74 45 Bootes 17, Kappa Bootis 4.6 6.6 13.4 bright white primary & bluish secondary 14 13.5 51 47 73 47 Bootes 21, Iota Bootis 4.9 7.5 38.5 wide yellow & blue pair 14 16.2 51 22 57 131 Bootes 29, Pi Bootis 4.9 5.8 5.6 fine bright white pair 14 40.7 16 25 64 115 Bootes 36, Epsilon Bootis (Izar) (*311) (HIP72105) 2.9 4.9 2.8 golden & greenish-blue 14 45 27 4 57 124 Bootes 37, Xi Bootis 4.7 7 6.6 beautiful yellow & reddish orange pair 14 51.4 19 6 62 86 Bootes 51, Mu Bootis 4.3 7 108.3 primary of yellow & close orange BC pair 15 24.5 37 23 62 96 Bootes Delta Bootis 3.5 8.7 105 wide yellow & blue pair 15 15.5 33 19 62 138 Bootes Arcturus -0.11 alignment star 14 15.6 19 10.4 56 162 Bootes NGC5248 galaxy with bright stellar nucleus and oval halo 13 37.5 8 53 72 56 Bootes NGC5676 elongated galaxy with slightly brighter center 14 32.8 49 28 72 59 Bootes NGC5689 galaxy, elongated streak with prominent oval core 14 35.5 48 45 16 330 Camelopardalis 1 Camelopardalis 5.7 6.8 10.3 white & -

Fixed Stars Interview with Diana K. Rosenberg by Edith Hathaway, USA
Fixed Stars Interview with Diana K. Rosenberg By Edith Hathaway, USA ob Hand calls Diana K. Rosenberg “the leading authority on Fixed Stars.” She has spent the past 30 years researching the subject of Fixed Stars. Her primary interest R has always been research and teaching, rather than the consulting side of astrology. Her preferred astrological techniques outside of the realm of Fixed Stars include Uranian Astrology (90 Degree Dial, Planetary Pictures, etc.), Derived Houses, Planetary Nodes, and Solstice Points. Diana has been a resident of New York City her entire life, while my residence in NYC spanned only 7 years, and I moved westwards in the USA in Feb. 1983. Our paths first crossed in 1982 when I was a student in Diana’s Uranian Astrology classes in Manhattan, NY. From 1988 to the early 1990s I made a gradual shift from Uranian (tropical) to Vedic (sidereal) astrology, a subject Diana always intended to study, but Fixed Stars came to take up all of her time. She has always been fascinated with very ancient things, and the study of antiquity. A different portion of this interview, mainly on Fixed Stars, is being published simultaneously by ISAR Journal (Journal of the International Society for Astrological Research), both print and On-line versions. See http://www.isarastrology.com. This larger interview at Saptarishis covers only a few pages of the same material, but also much more from Diana on stars. It segues at times into a dialogue between us about Fixed stars, mundane astrology, the Vedic nakshatras, and the two zodiacs – tropical and sidereal (items #12 through 14), also about her life and birth chart, including my commentary on her Vedic chart, at her request (item #16). -

The COLOUR of CREATION Observing and Astrophotography Targets “At a Glance” Guide
The COLOUR of CREATION observing and astrophotography targets “at a glance” guide. (Naked eye, binoculars, small and “monster” scopes) Dear fellow amateur astronomer. Please note - this is a work in progress – compiled from several sources - and undoubtedly WILL contain inaccuracies. It would therefor be HIGHLY appreciated if readers would be so kind as to forward ANY corrections and/ or additions (as the document is still obviously incomplete) to: [email protected]. The document will be updated/ revised/ expanded* on a regular basis, replacing the existing document on the ASSA Pretoria website, as well as on the website: coloursofcreation.co.za . This is by no means intended to be a complete nor an exhaustive listing, but rather an “at a glance guide” (2nd column), that will hopefully assist in choosing or eliminating certain objects in a specific constellation for further research, to determine suitability for observation or astrophotography. There is NO copy right - download at will. Warm regards. JohanM. *Edition 1: June 2016 (“Pre-Karoo Star Party version”). “To me, one of the wonders and lures of astronomy is observing a galaxy… realizing you are detecting ancient photons, emitted by billions of stars, reduced to a magnitude below naked eye detection…lying at a distance beyond comprehension...” ASSA 100. (Auke Slotegraaf). Messier objects. Apparent size: degrees, arc minutes, arc seconds. Interesting info. AKA’s. Emphasis, correction. Coordinates, location. Stars, star groups, etc. Variable stars. Double stars. (Only a small number included. “Colourful Ds. descriptions” taken from the book by Sissy Haas). Carbon star. C Asterisma. (Including many “Streicher” objects, taken from Asterism. -
Observing List
day month year Epoch 2000 local clock time: 23.98 Observing List for 23 8 2019 RA DEC alt az Constellation object mag A mag B Separation description hr min deg min 38 64 Andromeda Gamma Andromedae (*266) 2.3 5.5 9.8 yellow & blue green double star 2 3.9 42 19 50 84 Andromeda Pi Andromedae 4.4 8.6 35.9 bright white & faint blue 0 36.9 33 43 50 66 Andromeda STF 79 (Struve) 6 7 7.8 bluish pair 1 0.1 44 42 36 66 Andromeda 59 Andromedae 6.5 7 16.6 neat pair, both greenish blue 2 10.9 39 2 66 76 Andromeda NGC 7662 (The Blue Snowball) planetary nebula, fairly bright & slightly elongated 23 25.9 42 32.1 52 72 Andromeda M31 (Andromeda Galaxy) large sprial arm galaxy like the Milky Way 0 42.7 41 16 52 73 Andromeda M32 satellite galaxy of Andromeda Galaxy 0 42.7 40 52 53 72 Andromeda M110 (NGC205) satellite galaxy of Andromeda Galaxy 0 40.4 41 41 37 69 Andromeda NGC752 large open cluster of 60 stars 1 57.8 37 41 35 61 Andromeda NGC891 edge on galaxy, needle-like in appearance 2 22.6 42 21 67 81 Andromeda NGC7640 elongated galaxy with mottled halo 23 22.1 40 51 66 60 Andromeda NGC7686 open cluster of 20 stars 23 30.2 49 8 45 154 Aquarius 55 Aquarii, Zeta 4.3 4.5 2.1 close, elegant pair of yellow stars 22 28.8 0 -1 28 146 Aquarius 94 Aquarii 5.3 7.3 12.7 pale rose & emerald 23 19.1 -13 28 21 142 Aquarius 107 Aquarii 5.7 6.7 6.6 yellow-white & bluish-white 23 46 -18 41 36 187 Aquarius M72 globular cluster 20 53.5 -12 32 36 185 Aquarius M73 Y-shaped asterism of 4 stars 20 59 -12 38 33 143 Aquarius NGC7606 Galaxy 23 19.1 -8 29 37 184 Aquarius NGC7009 -

Audre Lorde Collection 1950-2002 Spelman College Archives
Audre Lorde Collection 1950-2002 Spelman College Archives Provenance The Audre Lorde Papers were donated to Spelman College in Lorde‘s will and received by the institution in 1995. Preferred Citation Published citations should take the following form: Identification of item, date (if known); Audre Lorde Papers; box number; folder number; Spelman College Archives. Restrictions Access Restrictions Open to researchers. Appointments are necessary for use of manuscript and archival materials. Use Restrictions Collection use is subject to all copyright laws. Permission to publish materials must be obtained in writing from the Director of Spelman College Archives. For more information, contact Descriptive Summary Creator Taronda Spencer, Brenda S. Banks and Kerrie Cotten Williams Title The Audre Lorde Papers Dates ca. 1950-2002 Quantity 40 linear ft. Biographical Note Poet, writer. Born Audre Geraldine Lorde on February 18, 1934, in New York, New York. Raised in New York, Lorde attended Hunter College. After graduating in 1959, she went on to get a master‘s degree in library science from Columbia University in 1961. Audre Lorde worked as a librarian in Mount Vernon, New York, and in New York City. She married attorney Edwin Rollins in 1962, and the couple had two children—Elizabeth and Jonathan. The couple later divorced. Lorde‘s professional career as a writer began in earnest in 1968 with the publication of her first volume of poetry, First Cities, was published in 1968. A second volume, Cables to Rage in 1970 was completed while Lorde was writer-in-residence at Tougaloo College in Mississippi. Lorde‘s third volume of poetry, From a Land Where Other People Live, written in 1973 was nominated for a National Book Award. -

A Search for Very High Energy Gamma Rays from PSR1706-44
\l^l-q( A Search for Very High EnergY Gamma Rays from PSR17O6-44 using the Atmospheric Õ.""ttkov Imagittg Technique A Thesis Submitted to the Department of Physics and Mathematical Physics University of Adelaide for the degree of Doctor of Philosophy by Gavin Peter Rowell B.Sc (Hons.) August 1995 Contents Abstract v Authorts Note vll Statement of Originality vlll Acknowledgements IX 1 An Overview of Gamma R^ay Astronomy 1 1.1 lntroduction t 1.2 Radio Pulsa¡s 3 1.3 High Energy 7-Ray Observations of Radio Pulsars 4 1.3.1 Previous Experiments 4 I.3.2 The Compton Gamma Ray Observatory 5 L.4 Significant Results in VHE ?-Ray Astronomy I 1.4.1 The Crab Nebula/Puisar 10 1.4.2 ùIarkarian 421 and 501 10 1.4.3 PSR 1706-44 12 1.5 Radio Pulsars as Sources of 7-Rays L2 1.5.1 ùIodels for Producing l{igh Energy 7-Rays T4 L.5.2 VHE-UHE l-Ray Production 77 2 Atmospheric Cerenkov Imaging Technique 19 2.1 Introduction . r9 2.2 Cosmic Ray EAS 20 2.2.I Energy Spectrum and Mass Composition 20 ,.), EÄS Properties 23 ôô4 ¿. /¿.t) Nrrclear component 24 2.2.4 lfuon Component 25 2.2.5 Electromagnetic Com ponent 26 ll CO¡TTENTS 2.3 Gamma Ray EAS 28 2.3.7 Lateral Distribution Ðo 2.4 Õerenkov Radiation Theory 30 2.5 Õerenkov Radiation from EAS 32 2.6 Lateral Distribution 34 2.7 Angu-lar Distribution and Image Formation 35 2.7.7 Simulations 36 2.7.2 Detector Development 39 2.8 Air Cerenkov Telescope Design and Optimisation 47 2.8.I Energy Threshold and Coilecting Area 43 2.8.2 DC Searches 44 2.8.3 Sea¡ches for Periodicity 45 2.9 Cosmic Ray Background Rejection 45 Tm¿ging 2.9.I Multi-Telescope . -

Dave Mitsky's Monthly Celestial Calendar
Dave Mitsky’s Monthly Celestial Calendar January 2010 ( between 4:00 and 6:00 hours of right ascension ) One hundred and five binary and multiple stars for January: Omega Aurigae, 5 Aurigae, Struve 644, 14 Aurigae, Struve 698, Struve 718, 26 Aurigae, Struve 764, Struve 796, Struve 811, Theta Aurigae (Auriga); Struve 485, 1 Camelopardalis, Struve 587, Beta Camelopardalis, 11 & 12 Camelopardalis, Struve 638, Struve 677, 29 Camelopardalis, Struve 780 (Camelopardalis); h3628, Struve 560, Struve 570, Struve 571, Struve 576, 55 Eridani, Struve 596, Struve 631, Struve 636, 66 Eridani, Struve 649 (Eridanus); Kappa Leporis, South 473, South 476, h3750, h3752, h3759, Beta Leporis, Alpha Leporis, h3780, Lallande 1, h3788, Gamma Leporis (Lepus); Struve 627, Struve 630, Struve 652, Phi Orionis, Otto Struve 517, Beta Orionis (Rigel), Struve 664, Tau Orionis, Burnham 189, h697, Struve 701, Eta Orionis, h2268, 31 Orionis, 33 Orionis, Delta Orionis (Mintaka), Struve 734, Struve 747, Lambda Orionis, Theta-1 Orionis (the Trapezium), Theta-2 Orionis, Iota Orionis, Struve 750, Struve 754, Sigma Orionis, Zeta Orionis (Alnitak), Struve 790, 52 Orionis, Struve 816, 59 Orionis, 60 Orionis (Orion); Struve 476, Espin 878, Struve 521, Struve 533, 56 Persei, Struve 552, 57 Persei (Perseus); Struve 479, Otto Struve 70, Struve 495, Otto Struve 72, Struve 510, 47 Tauri, Struve 517, Struve 523, Phi Tauri, Burnham 87, Xi Tauri, 62 Tauri, Kappa & 67 Tauri, Struve 548, Otto Struve 84, Struve 562, 88 Tauri, Struve 572, Tau Tauri, Struve 598, Struve 623, Struve 645, Struve -

The RBSE JOURNAL 2004
2004 The RBSE Journal The RBSE JOURNAL 2004 “Teacher Leaders in Research Based Science” (TLRBSE) is a Teacher Enhancement Program funded by the National Science Foundation. It consists of a distance learning course and a summer workshop for middle and high school teachers interested in incorporating leadership and research within their classes and school. TLRBSE brings the research experience to the classroom with materials, datasets, support, and mentors during the academic year. The RBSE Journal is an annual publication intended to present the research of students and teachers participating in the TLRBSE program. The RBSE Journal ii 2004 V1.3 Table of Contents SOLAR RESEARCH Horizontal Evershed Flow Velocities Of Ni I And Fe I Within Sunspots.................... 1 Cayle Castor and Matt Crevier Belmont High School, Belmont, New Hampshire Teacher: Thomas R. Morin TLRSBE 2003 Active Longitudes.............................................................................................................. 7 Danny Matarese, Lee Braun, and Michael Tiu Cranston High School East, Cranston, RI Teacher: Howard Chun, RBSE 1999 Sunspot Activity And Mars Exploration ...................................................................... 12 Michael TW Huang, Michael Paul St. Laurent, Michael Geary King Cranston High School East, Cranston, RI Teacher: Howard Chun, RBSE 1999 Magnetic Field Strength Comparisons Between Lone Sunspots And Clustered Sunspots Of Similar Size. ..............................................................................................