The Role of Submerged Macrophytes in Seasonally-Flowing Agricultural Streams
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Coastal Land and Groundwater for Horticulture from Gingin to Augusta
Research Library Resource management technical reports Natural resources research 1-1-1999 Coastal land and groundwater for horticulture from Gingin to Augusta Dennis Van Gool Werner Runge Follow this and additional works at: https://researchlibrary.agric.wa.gov.au/rmtr Part of the Agriculture Commons, Natural Resources Management and Policy Commons, Soil Science Commons, and the Water Resource Management Commons Recommended Citation Van Gool, D, and Runge, W. (1999), Coastal land and groundwater for horticulture from Gingin to Augusta. Department of Agriculture and Food, Western Australia, Perth. Report 188. This report is brought to you for free and open access by the Natural resources research at Research Library. It has been accepted for inclusion in Resource management technical reports by an authorized administrator of Research Library. For more information, please contact [email protected], [email protected], [email protected]. ISSN 0729-3135 May 1999 Coastal Land and Groundwater for Horticulture from Gingin to Augusta Dennis van Gool and Werner Runge Resource Management Technical Report No. 188 LAND AND GROUNDWATER FOR HORTICULTURE Information for Readers and Contributors Scientists who wish to publish the results of their investigations have access to a large number of journals. However, for a variety of reasons the editors of most of these journals are unwilling to accept articles that are lengthy or contain information that is preliminary in nature. Nevertheless, much material of this type is of interest and value to other scientists, administrators or planners and should be published. The Resource Management Technical Report series is an avenue for the dissemination of preliminary or lengthy material relevant the management of natural resources. -

Ministerial Decisions at at 12 October 2018
MINISTERIAL DECISIONS AS AT OCTOBER 2020 Recently received Awaiting decision pursuant to section 45(7) of Pending submission to Pending decision by Ministerial decision the Environmental Protection Act 1986 Minister for Aboriginal Affairs Minister for Aboriginal Affairs APPLICANT / MINISTERIAL LAND PURPOSE LANDOWNER DECISION September 2020 Lot 140 on DP 39512, CT 2227/905, 140 South Western Highway, Land Act No. 11238201, Lot 141 on DP 39512, CT 2227/906, 141 South Western Highway, Land Act No. 11238202, 202 Vittoria Road, Land Act No. 11891696, Glen Iris. Pending Intersection Vittoria Road Lot 201 on DP 57769, CT 2686/979, 201 submission to Main Roads South Western Highway South Western Highway, Land Act No. Minister for Western Australia upgrade and Bridge 0430 11733330, Lot 202 on DP 56668, CT Aboriginal Affairs replacement, Picton. 2754/978, Picton. Road Reserve, Land Act No.s 1575861, 11397280, 11397277, 1347375, and 1292274. Unallocated Crown Land, South Western Highway, Land Act No.s 11580413, 1319074 and 1292275, Picton. Pending Fortifying Mining Pty Ltd – Tenements M25/369, P25/2618, submission to Fortify Mining Pty Majestic North Project. To P25/2619, P25/2620, and P25/2621, Minister for Ltd undertake exploration and Goldfields. Aboriginal Affairs resource delineation drilling Reserve 34565, Lot 11835 on Plan Pending 240379, CT 3141/191, Coode Street, Landscape enhancement submission to City of South South Perth, Land Act No. 1081341 and and river restoration. To Minister for Perth Reserve 48325, Lot 301 on Plan 47451, construct the Waterbird Aboriginal Affairs CT 3151/548, 171 Riverside Drive, Land Refuge Act No. 11714773, Perth Pending Able Planning and Lot 501 on Plan 23800, CT 2219/673, submission to Lot 501 Yalyalup Urban Project 113 Vasse Highway, Yalyalup, Land Act Minister for Subdivision. -

Ludlow Titanium Minerals Mine, 34 Kilometres South of Bunbury
Ludlow Titanium Minerals Mine, 34 Kilometres South of Bunbury Cable Sands (WA) Pty Ltd Report and recommendations of the Environmental Protection Authority Environmental Protection Authority Perth, Western Australia Bulletin 1098 May 2003 ISBN. 0 7307 6734 5 ISSN. 1030 - 0120 Assessment No. 1385 Summary and recommendations Cable Sands (WA) Pty Ltd proposes to develop a mineral sands mine in a section of State Forest No.2, near Ludlow. This report provides the Environmental Protection Authority’s (EPA’s) advice and recommendations to the Minister for the Environment and Heritage on the environmental factors relevant to the proposal. Section 44 of the Environmental Protection Act 1986 requires the EPA to report to the Minister for the Environment and Heritage on the environmental factors relevant to the proposal and on the conditions and procedures to which the proposal should be subject, if implemented. In addition, the EPA may make recommendations as it sees fit. Relevant environmental factors The EPA decided that the following environmental factors relevant to the proposal required detailed evaluation in this report: (a) Tuart conservation; (b) Rehabilitation; and (c) Fauna. There were a number of other factors which were relevant to the proposal, but the EPA is of the view that the information set out in Appendix 3 provides sufficient evaluation. Conclusion The EPA has considered the proposal by Cable Sands (WA) Pty Ltd to develop a mineral sands mine in a section of State Forest No.2, 34 km south of Bunbury. Cable Sands (WA) Pty Ltd has worked hard with the community and government to develop an environmentally acceptable proposal in a region which has high significance for conservation. -

River Action Plan for the Sabina, Abba and Ludlow Rivers Vol 1. 2002
River Action Plan for the Sabina, Abba and Ludlow Rivers Volume 1. Maps and Recommendations for Sabina River and Woddidup Creek 2002 Soil and Land Conservation Natural Heritage Trust Council Western Australia GeoCatch River Action Plan for the Sabina, Abba and Ludlow Rivers Volume 1. Maps and Recommendations for Sabina River and Woddidup Creek 2002 Prepared for the Geographe Catchment Council - GeoCatch and the Vasse-Wonnerup Land Conservation District Committee by Genevieve Hanran-Smith Funded by the Natural Heritage Trust and the Water and Rivers Commission ISBN: 0-7309-7590-8 This report was prepared for GeoCatch, the Vasse-Wonnerup LCDC and landholders in the catchments of the Sabina, Abba and Ludlow Rivers. Sections 1 and 2 provide background information on the river action plan and the study area. Section 3 details the methodology used in assessing the condition of the rivers. Sections 4 and 5 outline the management issues identified and provide general management advice. Maps showing foreshore condition rating, fencing status, river features, management issues and weeds are included in Section 6 with specific management advice for each section of river. There are three volumes of this report. One for the Sabina River and Woddidup Creek, one for the Abba River, and one for the Ludlow River and Tiger Gully. Sections 1 to 5 are the same in all the reports. Section 6 differs in each volume and contains maps and specific management advice for each of the river systems. Figure 11 uses colour codes to show the foreshore conditions of the whole river system. It also provides an index to assist with locating specific sections of river. -

Rural Drainage Networks Vasse Taskforce | May 2015 |
Government of Western Australia Department of Water Factsheet: 3 Rural drainage networks Vasse Taskforce | May 2015 | Major drainage works in the Geographe Catchment commenced in the 1880s when the Capel River was diverted away from the Wonnerup Estuary into Geographe Bay through the Higgins cut. Over the past 100 years drainage works including the construction of surge barriers on the Vasse and Wonnerup estuaries, a network of small drains to remove water from farmland, river diversions and a series of large arterial drains, including the Vasse diversion drain, were undertaken. This has significantly altered the hydrology of the catchment. The drainage system enabled farming on the coastal plain that was previously inundated during winter and protected the growing town of Busselton from flooding. The combined effect of catchment clearing and major drainage works, however, also substantially reduced the capacity of the catchment to retain sediment and nutrients, greatly increasing nutrients and organic matter entering catchment waterways and Geographe Bay. The Water Corporation is the lead asset manager for the rural drainage network and will work closely with the Department of Water, which will lead the implementation of projects to improve water quality in catchment waterways through improved management of the rural drainage network. Key initiatives include: • new catchment water, flood and landform model • feasibility study into reconnecting rivers • drainage management plan Project Spotlight New catchment water, flood and landform model A range of options for improving water quality in waterways in the Geographe catchment has been raised over a number of years. This project will develop a whole of catchment water model and develop an estuarine model for the Vasse estuary to assess the feasibility of these and new innovative options for changing water flow in the catchment. -

The Distribution of Freshwater Fish in the South-Western Corner of Australia
The Distribution of Freshwater Fish in the South-Western Corner of Australia Report to Water and Rivers Commission David Morgan, Howard Gill & Ian Potter _;: ':1 Fish Research Group I ' ,, School of Biological imd Environmenta!~Ciences Murdoch University,,..,• ~ · Water and,:Rivers C~mmission Policy a!)li Planning Division / WATER REsOURCE TECHNICAL SERIES WATER AND RIVERS COMMISSION REPORT WRT4 1996 WATER AND RIVERS COMMISSION © Water and Rivers Commission of Western Australia, 1996 Published by the Water and Rivers Commission Hyatt Centre 3 Plain Street East Perth, Western Australia 6004 Telephone: (09) 278 0300 Publication Number: WRT4 ISBN 0-7309-7250-X STREAMLINE ABSTRACT This study investigates the distribution of freshwater fishes in the Busselton to Walpole Region. A total of 311 sites in 19 major catchments along the south-west coast from Capel to Walpole, were sampled using a variety of methods. New data was collated with that from previous studies to generate 15 species distribution maps. Habitat and life history notes and recommendations for conservation are made for each species. Changes in fish distribution are also commented upon. This study contributes to series of documents published for the purposes of water allocation planning in the Busselton to Walpole Region. Other publications focus on the following topics: • Recreational Use on Waterbodies in the Busselton- Walpole Region • Report on an Investigation into the Aboriginal Significance of Wetlands and Rivers in the Busselton-Walpole Region. • Enviromnental Significance of Wetlands and Rivers in the Busselton- Walpole Region • Historical Association of Wetlands and Rivers in the Busselton- Walpole Region. • Divertible Water Resources Key Words Water Resources Planning, Freshwater Fish Distribution, Wetland and Rivers, Busselton to Walpole, Western Australia. -

Information Sheet on Ramsar Wetlands (RIS) – 2009-2014 Version
Information Sheet on Ramsar Wetlands (RIS) – 2009-2014 version Available for download from http://www.ramsar.org/doc/ris/key_ris_e.doc and http://www.ramsar.org/pdf/ris/key_ris_e.pdf Categories approved by Recommendation 4.7 (1990), as amended by Resolution VIII.13 of the 8th Conference of the Contracting Parties (2002) and Resolutions IX.1 Annex B, IX.6, IX.21 and IX. 22 of the 9th Conference of the Contracting Parties (2005). 1. Name and address of the compiler of this form: FOR OFFICE USE ONLY. Western Australian Department of Conservation and Land DD MM YY Management (DCLM; now Department of Parks and Wildlife) in 1990. Updated by Roger Jaensch, Wetlands International – Oceania, on behalf of DCLM, in 1998, and by DCLM in 2000 and 2003. Updated by Wetland Research & Management on Designation date Site Reference Number behalf of Department of Environment and Conservation (now Department of Parks and Wildlife) in August 2007. Updated by Department of Parks and Wildlife in 2014. All inquiries should be directed to: Department of Parks and Wildlife Principal Coordinator, Wetlands Section 17 Dick Perry Avenue Kensington, WA, 6151 Australia Tel: +61-8-9219 9000 Email: [email protected] 2. Date this sheet was completed/updated: July 2014 3. Country: Australia 4. Name of the Ramsar site: The precise name of the designated site in one of the three official languages (English, French or Spanish) of the Convention. Alternative names, including in local language(s), should be given in parentheses after the precise name. Vasse-Wonnerup System. 5. Designation of new Ramsar site or update of existing site: The Vasse-Wonnerup System Ramsar site was designated on 7 June 1990. -

Appendix 5 Baseline Aquatic Biology and Water Quality Study
Cloverdale Project M-CV-00028 Baseline Aquatic Biology and Water Quality Study including Tiger Gully, Ludlow River and Capel River prepared for by Wetland Research & Management Cloverdale Project M-CV-00028 Baseline Aquatic Biology and Water Quality Study including Tiger Gully, Ludlow River and Capel River Prepared for: Iluka Resources Limited Level 23, 140 St Georges Terrace, Perth WA 6000 GPO Box U1988 Perth WA Ph (61 8) 9360 4700 By: Wetland Research & Management 28 William Street, Glen Forrest, WA 6071, Australia Ph (61 8) 9298 9807, Fax (61 8) 9380 1029, e-mail: awstorey@ cyllene.uwa.edu.au Draft Report April 2006 Frontispiece: (clockwise from main picture) Ludlow River, 1.2 km downstream from the Cloverdale project area; male koonac Cherax plebejus; nightfish Bostockia porosa. ii Study Team 8anagement: Sue Creagh Field Work: Sue Creagh & Jess Lynas Macroinvertebrate Identification: Lisa Chandler & Sue Creagh Data analysis: Sue Creagh & Andrew Storey Report: Sue Creagh Acknowledgements This project was undertaken by Wetland Research & Management (WRM) for Iluka Resources Limited. WRM would like to acknowledge Dr Don Edward (UWA) for assistance with Chironomidae taxonomy and Dr Mark Harvey (WAM) for Acarina taxonomy and Dr Rob Davis (Western Wildlife) for tadpole identifications. The maps of the study area were provided by Iluka Resources Limited. Shannon Jones (Iluka) is thanked for constructive criticism on the draft report and for her efficient overall management of this project on behalf of Iluka. The authors are grateful to Craig and Tom Hutton, Garry Bibby, Jan McKechnie and to the Norton, Armstrong, Weir and Whiteland families, who readily granted access to their pastoral properties. -

Iluka Resources Ltd. Cloverdale Project
Iluka Resources Ltd. Cloverdale Project Vegetation, Flora and Fauna Investigations Report April 2005 Contents 1. Introduction 1 1.1 Background 1 1.2 Scope of Work 1 2. Existing Environment – Desktop Assessment 3 2.1 Wetlands 3 2.2 Vegetation 3 2.3 Regional Significance of the Vegetation 5 2.4 Threatened Ecological Communities 5 2.5 Flora 6 2.6 Fauna 13 3. Results of Field Survey 18 3.1 Vegetation 18 3.2 Threatened Ecological Communities 21 3.3 Flora 21 3.4 Fauna 23 3.5 Fauna Habitat Assessment 24 4. Summary 25 5. References 26 Table Index Table 1 Vegetation Communities intersected by the Cloverdale Project. 4 Table 2 Threatened Ecological Communities found in the general Capel area 6 Table 3 Declared Rare and Priority Flora known from general Capel area 7 Table 4 Conservation Codes and Descriptions for CALM Declared Rare and Priority Flora Species. 12 Table 5 Listing of Potentially Occurring Significant, Rare and Priority Fauna Species – Cloverdale Prospect 17 Table 6 Vegetation Community Description and Condition Rating, Cloverdale Prospect Area 19 61/15996/49839 Cloverdale Project Vegetation, Flora and Fauna Investigations Table 7 Flora List per Site examined, Cloverdale Prospect 28 Table 8 List of Observed Fauna, Cloverdale Prospect 37 Figure Index Figure 1: Cloverdale Prospect Location Plan 2 Appendices A Flora List B Fauna List C Maps 61/15996/49839 Cloverdale Project Vegetation, Flora and Fauna Investigations 1. Introduction 1.1 Background Iluka Resources proposes to establish a mineral sands mine in the Tutunup area, called the Cloverdale Mineral Sands Project. The Cloverdale Project Area is sited approximately 8 kilometres south of Capel and 20 km east of Busselton, in the Shires of Busselton and Capel. -

Northern Territory
NORTHERN TERRITORY BAYVIEW 0820 CHARLES DARWIN 0820 COONAWARRA 0820 CULLEN BAY 0820 DARWIN INTERNATIONAL AIRPORT 0820 EAST POINT 0820 EATON 0820 FANNIE BAY 0820 LARRAKEYAH 0820 LUDMILLA 0820 PARAP 0820 RAAF BASE DARWIN 0820 STUART PARK 0820 THE GARDENS 0820 THE NARROWS 0820 WINNELLIE 0820 WOOLNER 0820 BAGOT 0820 DARWIN DC 0820 DARWIN MC 0820 WINNELLIE 0821 ACACIA HILLS 0822 ANGURUGU 0822 ANINDILYAKWA 0822 ANNIE RIVER 0822 BEES CREEK 0822 BELYUEN 0822 BLACK JUNGLE 0822 BLACKMORE 0822 BORDER STORE 0822 BURRUNDIE 0822 BYNOE 0822 BYNOE HARBOUR 0822 CAMP CREEK 0822 CHANNEL ISLAND 0822 CHARLES DARWIN 0822 CHARLOTTE 0822 CLARAVALE 0822 COBOURG 0822 COLLETT CREEK 0822 COOMALIE CREEK 0822 COX PENINSULA 0822 DALY 0822 DALY RIVER 0822 DARWIN RIVER DAM 0822 DELISSAVILLE 0822 DOUGLAS-DALY 0822 EAST ARM 0822 EAST ARNHEM 0822 ELRUNDIE 0822 EVA VALLEY 0822 FINNISS VALLEY 0822 FLEMING 0822 FLY CREEK 0822 FREDS PASS 0822 GALIWINKU 0822 GLYDE POINT 0822 GUNBALANYA 0822 GUNN POINT 0822 HAYES CREEK 0822 HIDDEN VALLEY 0822 HOTHAM 0822 HUGHES 0822 KAKADU 0822 KOOLPINYAH 0822 LAKE BENNETT 0822 LAMBELLS LAGOON 0822 LITCHFIELD PARK 0822 LIVINGSTONE 0822 LLOYD CREEK 0822 MANDORAH 0822 MANINGRIDA 0822 MAPURU 0822 MARANUNGA 0822 MARGARET RIVER 0822 MARRAKAI 0822 MCMINNS LAGOON 0822 MICKETT CREEK 0822 MIDDLE POINT 0822 MILIKAPITI 0822 MILINGIMBI 0822 MILYAKBURRA 0822 MINJILANG 0822 MOUNT BUNDEY 0822 MURRUMUJUK 0822 NAUIYU 0822 NEMARLUK 0822 NGANMARRIYANGA 0822 NUMBULWAR 0822 NUMBURINDI 0822 OENPELLI 0822 PALUMPA 0822 PEPPIMENARTI 0822 PIRLANGIMPI 0822 POINT STUART -
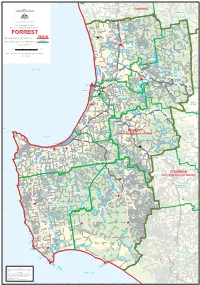
Map of the Division of Forrest
FORREST Lake Clifton Waroona OLD FORREST Yalgorup National WAROONA Hamel State Forest 2008 Park COAST CANNING COMMONWEALTH OF AUSTRALIA Hakea 20 BODDINGTON Yalgorup South Yalup Wagerup Willowdale Lower Dam Preston Beach SOUTH Yalup Brook Murray Williams Mt William RD Commonwealth Electoral Act 1918 River Yarloop River RD WESTERN River ELLIS STATE OF WESTERN AUSTRALIA Brook Map of the Commonwealth Electoral Division of Lake Brockman Bell Cookernup Harvey Moojelup Hoffman Lane Poole Reserve FORREST Harvey River Flats HWY Yamballup Harvey Names and Boundaries of Electoral Divisions PERTH 1 Warawarrup River Lake Preston KWINANA Korljekup Harvey Dam Names and Boundaries of Local Government Areas Harvey Tallanalla Map Number WA06/2008 20 Nalyern Lake Querilyup Stirling Dam Wokalup HARVEY Treesville Kilometres 0 5 10 15 Kilometres Harris Myalup River Wellesley River State Forest The Stinkwoods Flats Mornington SIMPLE CONIC PROJECTION WITH STANDARD PARALLELS 18° AND 36°S Yourdamung Lake Sheet 1 of 1 Binningup COLLIE Benger Lake Ballingall River RD Marbalup River Bingham Burragenup Beela Wellesley Brunswick Collie COLLIE Leschenault INDIAN OCEAN Hamilton - Fernbrook Creek Brunswick Junction WILLIAMS HWY Leschenault River Estuary Worsley Collie HWY Roelands River COALFIELDS River BYPASS Australind BYPASS 107 Cheetarra Allanson Gilfillan East Shenton Moorhead Ewington Collie Koombana Burekup Elbow Bay WESTERN COALFIELDS Eaton Waterloo Collie Buckingham Wilson Park Shotts BUNBURY River AUSTRALINDSOUTH RD 20 Collie Burn Cowcher 107 Wellington Dam -

Western Australia's Journal of Systematic Botany Issn 0085–4417
Nuytsia WESTERN AUSTRALIA'S JOURNAL OF SYSTEMATIC BOTANY ISSN 0085–4417 Lander, N.S. Elucidation of Olearia species related to O. paucidentata (Astereaceae: Astereae) Nuytsia 18: 83–95 (2008) All enquiries and manuscripts should be directed to: The Managing Editor – NUYTSIA Western Australian Herbarium Telephone: +61 8 9334 0500 Dept of Environment and Conservation Facsimile: +61 8 9334 0515 Locked Bag 104 Bentley Delivery Centre Email: [email protected] Western Australia 6983 Web: science.dec.wa.gov.au/nuytsia AUSTRALIA All material in this journal is copyright and may not be reproduced except with the written permission of the publishers. © Copyright Department of Environment and Conservation N.L.Nuytsia Lander, 18(1): Elucidation 83–95 (2008) of Olearia species related to O. paucidenta 83 Elucidation of Olearia species related to O. paucidentata (Asteraceae: Astereae) Nicholas S. Lander Western Australian Herbarium, Department of Environment and Conservation Locked Bag 104, Bentley Delivery Centre, Western Australia 6983 Abstract Lander, N.S. Elucidation of Olearia species related to O. paucidentata (Astereaceae: Astereae). Nuytsia 18(1): 83–95 (2008). A group of south-west Western Australian endemic species related to Olearia paucidentata (Steetz) F.Muell. ex Benth. are distinguished, and an identification key and comprehensive descriptions of each are presented. O. paucidentata is lectotypified. Eurybia lehmanniana Steetz is re-instated at specific rank asOlearia lehmanniana (Steetz) Lander. Maps and notes on typification, distributions, etc. of each species are provided. Introduction Prior to the preparation of an account of Olearia Moench (Asteraceae) in the ongoing Flora of Australia series it is necessary to clarify the limits of a number of Western Australian species described by Joachim Steetz (1845) which are easily confused and have been difficult to distinguish.