Phytosociological Indicators for Pheasant's Habitat Use
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ESTIMATION of PEAK HORIZONTAL ACCELERATIONS for the SITE of MUZAFFARABAD USING DETERMINISTIC APPROACH Mona Lisa1, Azam A
Pakistan Journal of Meteorology Vol. 1 Issue: 2, September, 2004 ESTIMATION OF PEAK HORIZONTAL ACCELERATIONS FOR THE SITE OF MUZAFFARABAD USING DETERMINISTIC APPROACH Mona Lisa1, Azam A. Khwaja† & Dr. Qamar-uz-Zaman Chaudhry2 Abstract: The site of Muzaffarabad is situated in the NW Himalayan Fold – and – Thrust Belt Pakistan, which occupies a 250 km wide and about 560 km long, irregularly shaped mountainous region stretching from the Afghan border near Parachinar, up to Kashmir Basin situated in the active zone of convergence thereby recording a large number of seismic events. Quite a large number of seismically active tectonic features like Main Mantle Thrust (MMT), Main Boundary Thrust (MBT), Mansehra Thrust, Jhelum Fault, Kotli Thrust, Raikot Fault, Thakot Fault, Riasi Thrust, Sangargali Thrust, Hissartang Fault and many others are located within this fold and thrust belt. Historical as well as Instrumental seismological data collected in the present study confirms the active nature of all these faults. In the present work, peak horizontal accelerations have been estimated for the site of Muzaffarabad using deterministic approach. The selection of this site has been based primarily due to the fact that this site is representing the location with heavy population, high seismicity and surrounded by active tectonic features. A total of twelve faults have been considered as a critical seismogenic features to the area and their maximum potential magnitudes and seven available attenuation equations, peak horizontal accelerations have been determined. On the basis of these maximum potential magnitudes and the peak horizontal accelerations, Main Boundary Thrust having peak horizontal accelerations of 0.47 g has been designated as the most hazardous for the site of Muzaffarabad. -

Diversity and Abundance of Medicinal Plants Among Different Forest-Use Types of the Pakistani Himalaya
DIVERSITY AND ABUNDANCE OF MEDICINAL PLANTS AMONG DIFFERENT FOREST-USE TYPES OF THE PAKISTANI HIMALAYA Muhammad Adnan (Born in Charsadda, Khyber Pakhtunkhwa, Pakistan) A Dissertation Submitted in Partial Fulfillment of the Requirements for the Academic Degree of Doctor of Philosophy (PhD) of the Faculty of Forest Sciences and Forest Ecology of the Georg-August-University of Göttingen Supervisor Prof. Dr. Dirk Hölscher Göttingen, November 2011 Reviewers Prof. Dr. Dirk Hölscher Prof. Dr. Christian Ammer Examiners Prof. Dr. Dirk Hölscher Prof. Dr. Christian Ammer Prof. Dr. Erwin Bergmeier ii SUMMARY Medicinal plants collected in the Himalayan forests are receiving increasing attention at the international level for a number of reasons and they play an important role in securing rural livelihoods. However, these forests have been heavily transformed over the years by logging, grazing and agriculture. This thesis examines the extent to which the diversity and abundance of medicinal plants are affected between forest-use types as a result of such transformations. In northwestern Pakistan we studied old-growth forest, degraded forests (forests degraded by logging, derived woodland, agroforest and degraded sites) and restored forests (re-growth forests and reforestation sites). An approximate map was initially established covering an area of 90 km2 of the studied forest-use types and fifteen and five plots were allocated to five and two forest-use types respectively at altitudes ranging from 2,200 m to 2,400 m asl. The abundance and diversity of medicinal plants were then assessed therein. Of the fifty-nine medicinal plant species (herbs and ferns) studied, old-growth forest contained the highest number thereof with fifty-five species, followed by re-growth forest with forty-nine species and finally, forest degraded by logging with only forty species. -

Status of Galliformes in Pipar Pheasant Reserve and Santel, Annapurna, Nepal
Status of Galliformes in Pipar Pheasant Reserve and Santel, Annapurna, Nepal 1 2 3 4 LAXMAN P. POUDYAL *, NAVEEN K. MAHATO , PARAS B. SINGH , POORNESWAR SUBEDI , HEM S. BARAL 5 and PHILIP J. K. MCGOWAN 6 1 Department of National Parks and Wildlife Conservation, PO Box 860, Babarmahal, Kathmandu, Nepal. 2 Red Panda Network – Nepal, PO Box 21477, Kathmandu, Nepal. 3 Biodiversity Conservation Society, PO Box 24304, Kathmandu, Nepal. 4 Department of Forests, Kathmandu, Nepal. 5 Bird Conservation Nepal, PO Box 12465, Lazimpat, Kathmandu, Nepal. 6 World Pheasant Association, Newcastle University Biology Field Station, Close House Estate, Heddon on the Wall, Newcastle upon Tyne, NE15 0HT UK. *Correspondence author - [email protected] Paper presented at the 4 th International Galliformes Symposium, 2007, Chengdu, China. Abstract The Galliformes of Pipar have been surveyed seven times between 1979 and 1998. The nearby area of Santel was surveyed using comparable methods in 2001. In continuance of the long- term monitoring at Pipar and to provide a second count at Santel, dawn call counts were conducted in both areas, using the same survey points as previous surveys, between 29 th April and 9 th May 2005. The aim of those surveys was to collect information on the status of pheasants and partridges and to look for any changes over time. In both areas, galliform numbers were higher in 2005 than in previous surveys, for most species. For each species there was no evidence of decline since the first counts were conducted nearly 30 years ago. Both areas provide good habitat for Galliformes and disturbance is not a serious issue. -

Phytotherapy Among the Rural Women of District Abbotabad
Pak. J. Bot., 45(SI): 253-261, January 2013. PHYTOTHERAPY AMONG THE RURAL WOMEN OF DISTRICT ABBOTABAD GHULAM MUJTABA SHAH*, ZAFAR JAMAL AND MANZOOR HUSSAIN Department of Botany Govt. Postgraduate College Abbottabad *Corresponding author’s e-mail: [email protected] Abstract The present communication highlights the scope of ethnomedicinal plants for women’s health care in Abbottabad district, Northern Pakistan. Participatory Action Research (PAR) and field visits were planned to elicit information on the uses of various medicinal plants by women. Field trips were undertaken covering different rural and tribal populated areas of the district to document ethnomedicinal plants used by women for the treatment of various diseases. The women chieftains were accorded a significant role in discussions since they possess more cognizances about the utility of local herbal products in curing various diseases. The study revealed that 67 plant species belonging to 65 genera and 47 families are used in women’s folk medicinal system. The medicinal plants are mostly used to cure amenorrhoea, skin allergies, and leucorrhoea, as abortifacient, post delivery pain, dandruff, eczema, tonic after delivery and for breast milk secretion. All these herbal medicines belong to 65.67% herbaceous ground flora, 8.95% shrubs, 22.38% trees and 2,98% climbers. Resins, exudates, leaves, shoots, fruits, seeds, bark, tubers and roots are the plants components which are utilized as medicinal ingredients. Plant components are used fresh, dried or both. Further research in needed to isolate the compounds responsible for the observed biological activity. Introduction been done in this field by many researchers (Chaudhri, 1959; Farooq, 1990; Haq & Hussain, 1993; Hussain & Plants have been used in various traditional medicinal Khaliq, 1996; Shinwari & khan, 1999; Gilani et al., 2001; systems for the treatment of human ailments. -

(Compositae-Inuleae) in Pakistan and Kashmir
Genus Inula L. (s. str.) (Compositae-Inuleae) in Pakistan and Kashmir RUBINA ABID & MOHAMMAD QAISER ABSTRACT ABID, R. & M. QAISER (2002). Genus Inula L. (s. str.) (Compositae-Inuleae) in Pakistan and Kashmir. Candollea 56: 315-325. In English, English and French abstracts. A revision of the genus Inula L. ( s. str .) in Pakistan and Kashmir is presented; eleven species are recognized including Inula stewartii Abid & Qaiser as a species new to science. In addition, key to the related genera and species, distribution, ecological notes and an illustration of the new spe - cies, are also provided. RÉSUMÉ ABID, R. & M. QAISER (2002). Le genre Inula L. (s. str.) (Compositae-Inuleae) au Pakistan et au Cachemire. Candollea 56: 315-325. En anglais, résumés anglais et français. Une révision du genre Inula L. ( s. str. ) est présentée. Onze espèces sont reconnues dont Inula ste - wartii Abid & Qaiser, espèce nouvelle pour la science. Une clé de détermination des genres affines et des espèces reconnues est fournie, ainsi que la distribution, des commentaires écologiques et une illustration de la nouvelle espèce. KEY-WORDS: Inula – COMPOSITAE – Pakistan – Kashmir. Introduction The genus Inula L. ( s. str. ) belongs to the tribe Inuleae of the family Compositae including about 100 species, mainly distributed in Europe, Africa and Asia. The genus Inula was described by LINNAEUS with thirteen species (1753, 1754). The pro - tologue used by LINNAEUS was quite generalized as he did not give the weightage to the finer details of floral characters. Later on, this genus was treated by variuos workers from time to time (viz., CANDOLLE, 1836; BENTHAM & HOOKER, 1873; BOISSIER, 1875; CLARKE, 1876; HOOKER, 1881; BECK, 1882; HOFFMANN, 1890; GORSCHKOVA, 1959; KITAMURA, 1960; GRIERSON, 1975; BALL & TUTIN, 1976; RECHINGER, 1980; ANDERBERG, 1991; KUMAR, 1995). -

A Note on Artificial Regeneration of Acacia
The Pakistan Journal of Forestry Vol.63(2), 2013 STATUS AND CONSERVATION OF PHEASANTS IN KAGHAN VALLEY Mian Muhammad Shafiq1 and Muhammad Saqib2 ABSTRACT Pheasants are considered the most beautiful birds in the world. Out of 49 species of pheasant found in the world five species i.e. Monal, (Lophoporus impejanus) koklass (Pucrasia macrolopha), Kalij (Lophura leucomelana), western horned Tragopan (Gragopan melanocephalus) and Cheer (Catreus wallichi) are found in Pakistan while four (4) species i.e. Monal, Koklass, Kalij and Western horned Tragopan are found in the study areas of Kaghan valley. This study was conducted in the Kaghan valley to know the status and conservation of pheasants. A questionnaire was designed and the villages were selected which were located near the reserve forest. A sample of 60 persons were interviewed in detail. The study revealed that the climate and topography of target area provides good habitat to pheasants, but impediments such as illegal hunting, poaching and human interference are the main causes for the decline in population. However declaration of some areas of the Kaghan valley as protected area (National park and wildlife sanctuary) has considerably contributed in the increase of pheasant population. The major earthquake in 2005 in the area had considerably decreased the population of pheasants as well as it has damaged the habitat of pheasants. It is recommended that there should be control on deforestation, habitat improvement and awareness raising campaign should also be carried out. INTRODUCTION Pheasants are the gallinaceous birds with beautiful, brilliant, multicolored and highly ornamental plumage. (Shah, 1987). Within the order Galliformes the pheasants comprise a very huge family with over 16 Genera amongst which there are 49 distinct species and sub species (13 occurring in sub continent) (IUCN, 1998). -

Life Forms, Leaf Size Spectra, Regeneration Capacity and Diversity of Plant Species Grown in the Thandiani Forests, District Abbottabad, Khyber Pakhtunkhwa, Pakistan
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Saudi Journal of Biological Sciences (2016) xxx, xxx–xxx King Saud University Saudi Journal of Biological Sciences www.ksu.edu.sa www.sciencedirect.com ORIGINAL ARTICLE Life forms, leaf size spectra, regeneration capacity and diversity of plant species grown in the Thandiani forests, district Abbottabad, Khyber Pakhtunkhwa, Pakistan Waqas Khan a, Shujaul Mulk Khan b,*, Habib Ahmad d, Abdulaziz A. Alqarawi c, Ghulam Mujtaba Shah a, Manzoor Hussain a, E.F. Abd_Allah c,e,* a Department of Botany, Hazara University Mansehra, KPK, Pakistan b Department of Plant Sciences, Quaid-i-Azam University, Islamabad, Pakistan c Department of Plant Production, Collage of Food & Agricultural Sciences, P.O. Box 2460, Riyadh 11451, Saudi Arabia d Islamia College University Peshawar, Pakistan e Seed Pathology Department, Plant Pathology Research Institute, ARC, Giza 12511, Egypt Received 26 August 2016; revised 8 November 2016; accepted 9 November 2016 KEYWORDS Abstract The life form and leaf size spectra of plant species of the Thandiani forests, district Life form; Abbottabad, were studied during the summer of 2013. These forests host 252 plant species of 97 Leaf spectra; families. Biological spectra showed that Hemicryptophytes (80 spp., 31.74%) were dominant fol- Diversity; lowed by Megaphanerophytes (51 spp., 20.24%), Therophytes (49 spp., 19.44%) and Nano- Forests; phanerophytes (45 spp., 17.86). Hemicryptophytes are the indicators of cold temperate Family importance values; vegetation. At the lower elevations, Megaphanerophytes and Nanophanerophytes were dominant Plant species which confirm trees as dominant habit form due to high soil depth, moisture and temperature fac- tors. -

MONITORING PHEASANTS (Phasianidae) in the WESTERN HIMALAYAS to MEASURE the IMPACT of HYDRO-ELECTRIC PROJECTS
THE RING 33, 1-2 (2011) DOI 10.2478/v10050-011-0003-7 MONITORING PHEASANTS (Phasianidae) IN THE WESTERN HIMALAYAS TO MEASURE THE IMPACT OF HYDRO-ELECTRIC PROJECTS Virat Jolli, Maharaj K. Pandit ABSTRACT Jolli V., Pandit M.K. 2011. Monitoring pheasants (Phasianidae) in the Western Himalayas to measure the impact of hydro-electric projects. Ring 33, 1-2: 37-46. In this study, we monitored pheasants abundance to measure the impact of a hydro- electric development project. The pheasants abundance was monitored using “call count” and line transect methods during breeding seasons in 2009-2011. Three call count stations and 3 transects were laid with varying levels of anthropogenic disturbance. To understand how the hydro power project could effect the pheasant population in the Jiwa Valley, we monitored it under two conditions; in the presence of hydro-electric project (HEP) con- struction and when human activity significantly declined. The Koklass Pheasant (Pucrasia macrolopha), Cheer Pheasant (Catreus wallichi) and Western Tragopan (Tragopan melano- cephalus) were not recorded in Manjhan Adit in 2009. During 2010 and 2011 springs, the construction activity was temporarily discontinued in Manjhan Adit. The pheasants re- sponded positively to this and their abundance increased near disturbed sites (Manjhan Adit). The strong response of pheasants to anthropogenic disturbance has ecological appli- cation and thus can be used by wildlife management in the habitat quality monitoring in the Himalayan Mountains. V. Jolli (corresponding author), M.K. Pandit, Centre for Inter-disciplinary Studies of Mountain and Hill Environment, Academic Research Building, Patel Road, Univ. of Delhi, Delhi, India, E-mail: [email protected] Key words: call count, anthropogenic disturbance, pheasant, monitoring, hydro-electric project INTRODUCTION Birds have been used extensively in environment and habitat quality monitoring. -

Site Office Ratti Gali Opposite Usmania Masjid Murree, Nathia Gali Road 0301 8593311 UAN:111-111-106
0301 8593311 UAN:111-111-106 Site Office Ratti Gali Opposite Usmania Masjid Murree, Nathia Gali Road DAM Address Come home to the fine embodiment of comfort and style, affordably priced to help you make your dream home a reality. Iman Heights invites you to experience the perfect balance of a residential community that meets your needs and budget, with the promise of a high quality lifestyle that you've always aspired for. Location & Surrounding Usmania Masjid Murree Nathia Gali Road Murree & the Galiyat The Murree Hills and the Gallies, 55 km from Islamabad, at an altitude of 8,200 ft. are the most popular summer resorts in Pakistan. Murree, known as the Queen of IMAN HEIGHTS Hills, is the most developed of these hill stations and has cool climate in summer and crispy cold in winter. Beyond Murree, the hill resorts of Ayubia, Khaira Gali, Bhurban, Patriata, Donga Gali and Nathia Gali offer cool respite from the torrid heat of plains. It offers a breathtaking view of high forested ridges and deep intersecting valleys with terraced slopes. In Patriata the Gondola cable cars, first of its kind in Pakistan, give an all round panoramic view of the valley. Mall Road Murree Murree's Mall Road is famous small stretch of road having number of shops and restaurant. It's popular among tourist around the country and all over the world. Mall Road is the busiest part of Murree where visitors love walking while doing shopping and buying from handicrafts to dry fruits and from shawls to shoes. Patriata Chair Lift Patriata is a great created mountain resort having tall trees with beautiful green hills. -

Impact of Transport Network Changes on Tourism in Protected Areas: a Case Study of Ayubia National Park, Pakistan
Journal of Transportation Technologies, 2020, 10, 325-350 https://www.scirp.org/journal/jtts ISSN Online: 2160-0481 ISSN Print: 2160-0473 Impact of Transport Network Changes on Tourism in Protected Areas: A Case Study of Ayubia National Park, Pakistan Muhammad Shaker*, Muhammad Adnan, Elke Hermans, Ansar Yasar, Geert Wets UHasselt - Universiteit Hasselt, Transportation Research Institute (IMOB), Diepenbeek, Belgium How to cite this paper: Shaker, M., Ad- Abstract nan, M., Hermans, E., Yasar, A. and Wets, G. (2020) Impact of Transport Network The inflow caused by tourists in peak seasons exerts an uncontrollable pres- Changes on Tourism in Protected Areas: A sure on the existing infrastructure. The Ayubia National Park in Pakistan Case Study of Ayubia National Park, Pa- faces traffic delays and capacity restraints on the connecting roads in peak kistan. Journal of Transportation Technol- ogies, 10, 325-350. season. The study focuses on the formulation of critical strategies by deploy- https://doi.org/10.4236/jtts.2020.104021 ing amendments in the transport network. The methodology contains three parts: 1) a questionnaire was designed to inquire about several variables from Received: August 29, 2020 Accepted: September 25, 2020 the visitors; 2) the second part was traffic count data collection and analysis. Published: September 28, 2020 Based on the response collected, the impact of multiple strategies on the net- work was analyzed using TransCad; 3) in the third part, the results obtained Copyright © 2020 by author(s) and were shared with experts to gain their valuable opinions. It was observed that Scientific Research Publishing Inc. This work is licensed under the Creative the time of the day based access restriction to heavy vehicles could lead to Commons Attribution International dropping the Volume to capacity ratio from 1.7 to 1.2. -
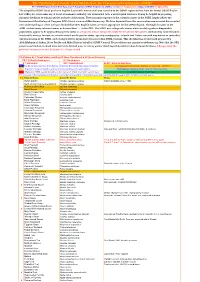
Simplified-ORL-2019-5.1-Final.Pdf
The Ornithological Society of the Middle East, the Caucasus and Central Asia (OSME) The OSME Region List of Bird Taxa, Part F: Simplified OSME Region List (SORL) version 5.1 August 2019. (Aligns with ORL 5.1 July 2019) The simplified OSME list of preferred English & scientific names of all taxa recorded in the OSME region derives from the formal OSME Region List (ORL); see www.osme.org. It is not a taxonomic authority, but is intended to be a useful quick reference. It may be helpful in preparing informal checklists or writing articles on birds of the region. The taxonomic sequence & the scientific names in the SORL largely follow the International Ornithological Congress (IOC) List at www.worldbirdnames.org. We have departed from this source when new research has revealed new understanding or when we have decided that other English names are more appropriate for the OSME Region. The English names in the SORL include many informal names as denoted thus '…' in the ORL. The SORL uses subspecific names where useful; eg where diagnosable populations appear to be approaching species status or are species whose subspecies might be elevated to full species (indicated by round brackets in scientific names); for now, we remain neutral on the precise status - species or subspecies - of such taxa. Future research may amend or contradict our presentation of the SORL; such changes will be incorporated in succeeding SORL versions. This checklist was devised and prepared by AbdulRahman al Sirhan, Steve Preddy and Mike Blair on behalf of OSME Council. Please address any queries to [email protected]. -

District Profile ABBOTTABAD
District Profile ABBOTTABAD Prepared By SMEDA, NWFP Small & Medium Enterprises Development Authority Ministry of Industries & Production Government of Pakistan February 2009 Table of Contents S. Contents Page No No 1 Introduction……………………………………………….…… ………1 2 History…………………………………………………….…..... ………2 3 Economic Scenario of the district…………………….…..... ………3 4 Economic Potential……………………………………….……. ………4 4.1 Agriculture/Horticulture.……………………………….…. ………4 4.2 Forestry ……………………………………………….…... ………4 4.3 Livestock and poultry development.………….…………… ………5 4.4 Fishery ……………………………………………….….... ………5 4.5 Mineral……………………………………………………. ………6 5 Tourism………………………………………………………… ………7 a) Ayubia ……………………….…....................... ………7 b) Dungagali…………………………………….…. ………8 c) Nathiagali…………………………….................. ………8 d) Thandiani……………………………….............. ………8 6 Industry………………………………………………………… ………9 7 Clusters………………………………………………………… ……..10 Introduction……………………………………………. ……..10 Product range……………………………………………. …..…11 8 Trade and trade centers……………………………………….. …..…11 9 Small Investment Projects for the District…………………… …..…12 9.1 Trout Farming……………………………………………. ……..13 9.2 Crochet/Embroidery Stitching Unit ……….………..…... ….….15 9.3 Poultry Farm………….………………………………….. …......16 9.4 Honey Bee Keeping……………………………………… ……..17 9.5 Walk In Tunnel Vegetables Farm……………………….. ……..18 1. Introduction: Abbottabad is a district in the North-West Frontier Province of Pakistan. The district covers an area of 1,969 km with the city of Abbottabad being the principal town of this district. Neighbouring