Spectra Libary Index Ichem/Aldrich ATR Library
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Electrochemical and Quantum Chemical Studies on Corrosion Inhibition Performance Of
Materials Research. 2020; 23(2): e20180610 DOI: https://doi.org/10.1590/1980-5373-MR-2018-0610 Electrochemical and Quantum Chemical Studies on Corrosion Inhibition Performance of 2,2’-(2-Hydroxyethylimino)bis[N-(alphaalpha-dimethylphenethyl)-N-methylacetamide] on Mild Steel Corrosion in 1M HCl Solution Iman Danaeea* , S. RameshKumarb, M. RashvandAveic, M. Vijayand aPetroleum University of Technology, Abadan Faculty of Petroleum Engineering, Abadan, Iran bSri Vasavi College, Department of Chemistry, Erode, Tamilnadu-638 316, India. cK. N. Toosi University of Technology, Department of chemistry, Tehran, Iran dCentral Electrochemical Research Institute, Centre for Conducting Polymers, Electrochemical Materials Science Division, Karikudi, 630006, India Received: September 7, 2018; Revised: January 13, 2020; Accepted: March 16, 2020 The inhibitory effect of Oxethazaine drug, 2,2’-(2-Hydroxyethylimino)bis[N-(alphaalpha- dimethylphenethyl)-N-methylacetamide] on corrosion of mild steel in 1M HCl solution was studied by weight loss measurements, electrochemical impedance spectroscopy and potentiodynamic polarization methods. The results of gravimetric and electrochemical methods demonstrated that the inhibition efficiency increased with an increase in inhibitor concentration in 1M HCl solution. The results from electrochemical impedance spectroscopy proved that the inhibition action of this drug was due to adsorption on the metal surface. Potentiodynamic polarization studies revealed that the molecule was a mixed type inhibitor. The adsorption of the molecule on the metal surface was found to obey Langmuir Adsorption isotherm. Potential of zero charge at the metal-solution interface was measured to provide the inhibition mechanism. The temperature dependence of the corrosion rate was also studied in the temperature range from 30 to 50 °C. Quantum chemical calculations were applied to correlate electronic structure parameters of the drug with its inhibition performance. -

WO 2008/096257 Al
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (43) International Publication Date PCT (10) International Publication Number 14 August 2008 (14.08.2008) WO 2008/096257 Al (51) International Patent Classification: 500038 (IN). BURIDIPADU, Sunil Kumar [IN/IN]; C07D 239/42 (2006.01) Aurobindo Pharma Limited, Plot No.2, Maitrivihar, Ameerpet, Andhra Pradesh, Hyderabad 500038 (IN). (21) International Application Number: MEENAKSHISUNDERAM, Sivakumaran [IN/IN]; PCT/IB2008/000290 Aurobindo Pharma Limited, Plot No.2, Maitrivihar, Ameerpet, Andhra Pradesh, Hyderabad 500038 (IN). (22) International Filing Date: 4 February 2008 (04.02.2008) (81) Designated States (unless otherwise indicated, for every (25) Filing Language: English kind of national protection available): AE, AG, AL, AM, AO, AT,AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, CA, (26) Publication Language: English CH, CN, CO, CR, CU, CZ, DE, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, (30) Priority Data: IL, IN, IS, JP, KE, KG, KM, KN, KP, KR, KZ, LA, LC, 277/CHE/2007 8 February 2007 (08.02.2007) IN LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, 1121/CHE/2007 29 May 2007 (29.05.2007) IN MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PG, PH, PL, PT, RO, RS, RU, SC, SD, SE, SG, SK, SL, SM, SV, (71) Applicant (for all designated States except US): AU- SY, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ROBINDO PHARMA LIMITED [IN/IN]; Plot No.2, ZA, ZM, ZW Maitrivihar, Ameerpet, Andhra Pradesh, Hyderabad 500038 (IN). -

Fullerene-Acene Chemistry
University of New Hampshire University of New Hampshire Scholars' Repository Doctoral Dissertations Student Scholarship Spring 2007 Fullerene-acene chemistry: Part I Studies on the regioselective reduction of acenes and acene quinones; Part II Progress toward the synthesis of large acenes and their Diels-Alder chemistry with [60]fullerene Andreas John Athans University of New Hampshire, Durham Follow this and additional works at: https://scholars.unh.edu/dissertation Recommended Citation Athans, Andreas John, "Fullerene-acene chemistry: Part I Studies on the regioselective reduction of acenes and acene quinones; Part II Progress toward the synthesis of large acenes and their Diels-Alder chemistry with [60]fullerene" (2007). Doctoral Dissertations. 363. https://scholars.unh.edu/dissertation/363 This Dissertation is brought to you for free and open access by the Student Scholarship at University of New Hampshire Scholars' Repository. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of University of New Hampshire Scholars' Repository. For more information, please contact [email protected]. FULLERENE-ACENE CHEMISTRY: PART I: STUDIES ON THE REGIOSELECTIVE REDUCTION OF ACENES AND ACENE QUINONES; PART II: PROGRESS TOWARD THE SYNTHESIS OF LARGE ACENES AND THEIR DIELS- ALDER CHEMISTRY WITH [60]FULLERENE VOLUME 1 CHAPTERS 1-5 BY ANDREAS JOHN ATHANS B.S. University of New Hampshire, 2001 DISSERTATION Submitted to the University of New Hampshire in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy m Chemistry May, 2007 Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. UMI Number: 3 2 6 0 5 8 6 INFORMATION TO USERS The quality of this reproduction is dependent upon the quality of the copy submitted. -

The Development of Simplified Microreactors for Use in Multi-Step Reaction Sequences
THE DEVELOPMENT OF SIMPLIFIED MICROREACTORS FOR USE IN MULTI-STEP REACTION SEQUENCES A Dissertation Presented to the Faculty of the Graduate School of Cornell University In Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy by Andrew R. Bogdan August 2009 © 2009 Andrew R. Bogdan THE DEVELOPMENT OF SIMPLIFIED MICROREACTORS FOR USE IN MULTI-STEP REACTION SEQUENCES Andrew R. Bogdan, Ph. D. Cornell University 2009 This dissertation describes the development of simplified microreactors to be used in multi-step reaction sequences. Microreactors are a developing technology with numerous benefits and whose modular design permits multi-step reaction sequences to be performed in a continuous flow process. The first chapter discusses the physical properties of microreactors and describes how performing reactions in these miniaturized reactors leads to safer, more efficient and more selective chemical transformations. A brief discussion of multi-step reaction sequences already performed in flow will also be presented. These multi-step reaction sequences, however, rarely rely on catalysis to facilitate chemical transformations. This area therefore has room for great improvement. The next two chapters discuss the development and application of solid-supported catalysts to be used in flow. In the first of these two chapters, two catalysts are immobilized on a solid-support and used in a simplified flow reactor. These reactions proved to be more efficient than similar batch reaction and the catalysts were highly recyclable. The next chapter presents a supported TEMPO catalyst used to oxidize alcohols to carbonyl species. These three catalysts set the foundation for multi-step flow reaction to be potentially performed in the group. -

Durham E-Theses
Durham E-Theses Molecular pharmacology of a Novel NR2B-selective NMDA Receptor Antagonist Bradford, Andrea Marie How to cite: Bradford, Andrea Marie (2006) Molecular pharmacology of a Novel NR2B-selective NMDA Receptor Antagonist, Durham theses, Durham University. Available at Durham E-Theses Online: http://etheses.dur.ac.uk/2736/ Use policy The full-text may be used and/or reproduced, and given to third parties in any format or medium, without prior permission or charge, for personal research or study, educational, or not-for-prot purposes provided that: • a full bibliographic reference is made to the original source • a link is made to the metadata record in Durham E-Theses • the full-text is not changed in any way The full-text must not be sold in any format or medium without the formal permission of the copyright holders. Please consult the full Durham E-Theses policy for further details. Academic Support Oce, Durham University, University Oce, Old Elvet, Durham DH1 3HP e-mail: [email protected] Tel: +44 0191 334 6107 http://etheses.dur.ac.uk 2 Molecular Pharmacology of a Novel NR2B-รelective NMDA Receptor Antagonist PhD Thesis The copyright of this thesis rests with the author or ¿he university to which it was submitted. No quotation from it, or ๒fonnation derived from it may be published without the prior written consent of the author or university, and any information derived from it should be acknowledged. Andrea Marie Bradford School of Biological & Вю^ Sciences University of Durham Supervisor: Dr Paul Chazot October 2006 7 JUM 200? Abstract The NMDA receptor is a heteromeric ligand-gated ion channel in the central nervous system (CNS). -

United States Patent Office Paterated Feb
3,306,895 United States Patent Office Paterated Feb. 28, 1967 1. 2 3,306,895 The compounds of this invention are obtained by treat HETEROCYCLIC DERVATIVES OF TRPHENYL ment of triphenylethylene derivatives of the type: , ETHYLENES, TRIPHENYLETHANES AND TRI PHENYLETHANOLS Edward McCreery Roberts, George Philip Claxton, and 5 Frances Gertrude Fallon, Cincinnati, Ohio, assignors to Richardson-Merrell Inc., New York, N.Y., a corpo ration of Delaware No Drawing. Filed June 27, 1962, Ser. No. 205,551 10 Claims. (C. 260-240) k This invention relates to a series of novel and useful O where R and R' are as previously defined, with an ap compounds and processes for preparing the same. More propriate organometallic derivative such as cy-picolyl particularly, this invention relates to a series of triphenyl lithium (as in Examples 2 and 5), 3-pyridyl lithium (as ethanes, triphenylethylenes, and triphenylethanols, in in Example 6), or 2-pyridyl lithium (as in Examples 1 which one of the phenyl groups is substituted with a pyri and 4). Such treatment is conveniently carried out by dyl or reduced pyridyl ring which is separated from the mixing the reactants in an inert solvent such as ether, aromatic portion of the molecule by an oxygenated alkyl benzene, toluene, or combinations of such inert solvents fragment of one, two or three carbon atoms. The inven at appropriate temperatures chosen in the range of tion also includes nontoxic, water-soluble addition salts, -60° C. to 80° C. The resulting imine is hydrolyzed to quaternary ammonium derivatives and N-oxide deriva the corresponding ketone without being isolated by being tives of the new compounds. -

Sulfolane: Magic Extractor Or Bad Actor? Pilot-Scale Study on Solvent Corrosion Potential
sustainability Review Sulfolane: Magic Extractor or Bad Actor? Pilot-Scale Study on Solvent Corrosion Potential Andrzej Bak 1,* , Violetta Kozik 1, Paulina Dybal 1, Slawomir Kus 2, Aleksandra Swietlicka 1 and Josef Jampilek 3,* 1 Department of Synthesis Chemistry, Faculty of Mathematics, Physics and Chemistry, University of Silesia, Szkolna 9, 40007 Katowice, Poland; [email protected] (V.K.); [email protected] (P.D.); [email protected] (A.S.) 2 Honeywell Process Solutions, 11201 Greens Crossing Blvd, Suite 700 Houston, TX 77067, USA; [email protected] 3 Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Comenius University, Odbojarov 10, 83232 Bratislava, Slovakia * Correspondence: [email protected] (A.B.); [email protected] (J.J.); Tel.: +48-32-359-1197 (A.B.) Received: 17 September 2018; Accepted: 11 October 2018; Published: 14 October 2018 Abstract: The sulfur-containing derivatives and their metabolites, regarded as ‘old devils of green’ chemistry, constitute a relevant class of air/water/soil contaminants in over-polluted world. In fact, some industrially-engineered solvents have become environmentally unfavorable. An attractive alternative to commonly used industrial liquids is sulfolane (C4H8SO2), an anthropogenic medium. The main objective of this paper is the comprehensive review focusing mainly on the state-of-the-art aspects of the sulfolane synthesis, application of sulfolane as an extractive solvent due to its ‘unique’ physicochemical properties as well as the potential of sulfolane to cause equipment corrosion and subsequent spills. The potential risk for groundwater contamination, danger for human health and ways of sulfolane biodegradation were briefly reviewed as well. Interestingly, the analysis performed on data stored in the Reaxys database revealed an alternating tendency of waxing and waning interest in sulfolane during the space of the last fifty years. -

Octahydroquinoxalin-2 (1H)-One-Based Aminophosphonic
materials Article Octahydroquinoxalin-2(1H)-One-Based Aminophosphonic Acids and Their Derivatives—Biological Activity towards Cancer Cells Jakub Iwanejko 1 , El˙zbietaWojaczy ´nska 1,* , Eliza Turlej 2 , Magdalena Maciejewska 2 and Joanna Wietrzyk 2 1 Faculty of Chemistry, Wrocław University of Science and Technology, Wybrze˙zeWyspia´nskiego 27, 50-370 Wrocław, Poland; [email protected] 2 Department of Experimental Oncology, Hirszfeld Institute of Immunology and Experimental Therapy, Polish Academy of Sciences, Rudolfa Weigla 12, 53-114 Wrocław, Poland; [email protected] (E.T.); [email protected] (M.M.); [email protected] (J.W.) * Correspondence: [email protected]; Tel.: +48-71-320-2410 Received: 19 March 2020; Accepted: 19 May 2020; Published: 22 May 2020 Abstract: In the search for new antitumor agents, aminophosphonic acids and their derivatives based on octahydroquinoxalin-2(1H)-one scaffold were obtained and their cytotoxic properties and a mechanism of action were evaluated. Phosphonic acid and phosphonate moieties increased the antiproliferative activity in comparison to phenolic Mannich bases previously reported. Most of the obtained compounds revealed a strong antiproliferative effect against leukemia cell line (MV-4-11) with simultaneous low cytotoxicity against normal cell line (mouse fibroblasts-BALB/3T3). The most active compound was diphenyl-[(1R,6R)-3-oxo-2,5-diazabicyclo[4.4.0]dec-4-yl]phosphonate. Preliminary evaluation of the mechanism of action showed the proapoptotic effect associated with caspase 3/7 induction. Keywords: aminophosphonate; imine; antiproliferative activity; cell cycle; mitochondrial membrane potential 1. Introduction At present, our attention is focused on the COVID-19 pandemic and there is a tendency to neglect the civilization diseases which however remain the main reason for mortality worldwide. -

Improving Thermodynamic Consistency Among Vapor Pressure, Heat of Vaporization, and Liquid and Ideal Gas Heat Capacities Joseph Wallace Hogge Brigham Young University
Brigham Young University BYU ScholarsArchive All Theses and Dissertations 2017-12-01 Improving Thermodynamic Consistency Among Vapor Pressure, Heat of Vaporization, and Liquid and Ideal Gas Heat Capacities Joseph Wallace Hogge Brigham Young University Follow this and additional works at: https://scholarsarchive.byu.edu/etd Part of the Chemical Engineering Commons BYU ScholarsArchive Citation Hogge, Joseph Wallace, "Improving Thermodynamic Consistency Among Vapor Pressure, Heat of Vaporization, and Liquid and Ideal Gas Heat Capacities" (2017). All Theses and Dissertations. 6634. https://scholarsarchive.byu.edu/etd/6634 This Dissertation is brought to you for free and open access by BYU ScholarsArchive. It has been accepted for inclusion in All Theses and Dissertations by an authorized administrator of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. Improving Thermodynamic Consistency Among Vapor Pressure, Heat of Vaporization, and Liquid and Ideal Gas Heat Capacities Joseph Wallace Hogge A dissertation submitted to the faculty of Brigham Young University In partial fulfillment of the requirements for the degree of Doctor of Philosophy W. Vincent Wilding, Chair Thomas A. Knotts Dean Wheeler Thomas H. Fletcher John D. Hedengren Department of Chemical Engineering Brigham Young University Copyright © 2017 Joseph Wallace Hogge All Rights Reserved ABSTRACT Improving Thermodynamic Consistency Among Vapor Pressure, Heat of Vaporization, and Liquid and Ideal Gas Heat Capacity Joseph Wallace Hogge Department of Chemical Engineering, BYU Doctor of Philosophy Vapor pressure ( ), heat of vaporization ( ), liquid heat capacity ( ), and ideal gas heat capacity ( ) are important properties for process design and optimization. This work vap Δvap focuses on improving the thermodynamic consistency and accuracy of the aforementioned properties since these can drastically affect the reliability, safety, and profitability of chemical processes. -
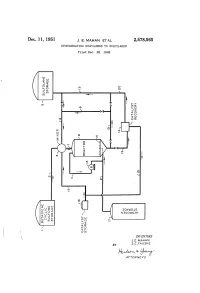
Rudo-At-Thur a 77OAPAVA 1S Patented Dec
Dec. 11, 1951 J. E. MAHAN ET AL 2,578,565 HYDROGENATING SULFOLENES TO SULFOLANES Filed Dec. 28, 1948 o g X g t 92.9 DVOS a dig NGOOOH Ofse INVENTORS J.E. MAHAN BY S.C. FAUSKE Rudo-at-thur A 77OAPAVA 1S Patented Dec. 11, 1951 2,578,565 UNITED STATES PATENT OFFICE 2,578,565 HYDROGENATING SULFOLENESTO SULFOLANES John E. Mahan and Sig C. Fauske, Bartlesville, Okla., assignors to Phillips Petroleum Company, a corporation of Delaware Application December 28, 1948, Serial No. 67,745 15 Claims. (CI. 260-332.1) 1. 2 This invention relates to the production of the generic term “a sulfolane' or "a Sulfolane Sulfolanes by the hydrogenation of the corre compound' covering not only the above Com sponding unsaturated Sulfolenes. In One par pound but also the substituted derivatives there ticular embodiment it relates to an improved of, particularly those in which various radicals process for the production of 2,3,4,5-tetrahy mentioned in the preceding paragraph are Sub drothiophene-1,1-dioxide, commercially known stituted for one or more of the hydrogen atoms as sulfolane, by the catalytic hydrogenation of of the above structure. Where such a radical the corresponding unsaturated cyclic butadiene is hydrogenatable under the conditions of our monosulfone, i. e. 2,5-dihydrothiophene-1,1- process, it will be understood that the sulfolane dioxide, commercially known as Sulfolene, in the 0. containing the hydrogenated radical is included presence of a novel solvent. when reference is made to a sulfolane compound The term “a sulfolene compound' as employed which “corresponds' to a given sulfolene com herein and in the appended claims, defines ge pound. -

|||||||III US005514680A United States Patent (19) 11 Patent Number: 5,514,680 Weber Et Al
|||||||III US005514680A United States Patent (19) 11 Patent Number: 5,514,680 Weber et al. (45) Date of Patent: May 7, 1996 54 GLYCINE RECEPTOR ANTAGONISTS AND 0377112 7/1990 European Pat. Off.. THE USE THEREOF 0511152 10/1992 European Pat. Off.. 572852 12/1993 European Pat. Off.. 2451049 4/1976 Germany. (75) Inventors: Eckard Weber, Laguna Beach, Calif.; 2446543 4/1976 Germany. John F. W. Keana, Eugene, Oreg. 2847285 5/1980 Germany. 72674 11/1974 Poland. 73) Assignees: State of Oregon, acting by and WO91/13878 9/1991 WIPO. through The Oregon State Board of WO92/07847 11/1991 WIPO. Higher Education, acting for and on WO92/O2487 2/1992 WIPO. behalf of The Oregon Health Sciences WO92/11012 7/1992 WIPO. University and The University of WO92/11245 7/1992 WIPO. Oregon, Eugene, Oreg.; The Regents WO92/14740 9/1992 WIPO : of the University of California, WO92/15565 9/1992 WIPO. Oakland, Calif. 94.07500 4/1994 WIPO. OTHER PUBLICATIONS (21) Appl. No.: 148,259. Allison et al., Polyfluoroheterocyclic Compounds, Part XX. 22 Filed: Nov. 5, 1993 Preparation and Nucleophilic Substitution of Hexafluoro quinoxaline, J. Fluorine Chem. 1:59-667 (1971). Related U.S. Application Data Burton et al., Halogeno-o-phenylenediamines and Derived Heterocycles. Part I. Reductive Fission of Benzotriazoles to 63 Continuation-in-part of PCT/US93/05859, Jun. 17, 1993, o-Phenylenediamines, J. Chem. Soc. (C) 10:1268-1273 which is a continuation-in-part of Ser. No. 69,274, May 28, (1968). 1993, abandoned, which is a continuation-in-part of Ser. No. Lutfy et al., Blockade of Morphine Tolerance by 995,167, Dec. -

Synthesis and Characterization of Thermoplastic
SYNTHESIS AND CHARACTERIZATION OF THERMOPLASTIC POLYPHENOXYQUINOXALINES A Dissertation Presented to The Graduate Faculty of the University of Akron In Partial Fulfillment of the Requirement for the Degree Doctor of Philosophy Haci Bayram Erdem May, 2008 © 2008 HACI BAYRAM ERDEM ALL RIGHTS RESERVED SYNTHESIS AND CHARACTERIZATION OF THERMOPLASTIC POLYPHENOXYQUINOXALINES Haci Bayram Erdem Dissertation Approved: Accepted: ______________________________ _____________________________ Advisor Department Chair Frank W. Harris Mark D. Foster ______________________________ _____________________________ Committee Member Dean of the College Stephen Z. D. Cheng Stephen Z. D. Cheng ______________________________ _____________________________ Committee Member Dean of the Graduate School Judit Puskas George R. Newkome ______________________________ _____________________________ Committee Member Date Roderic P. Quirk ______________________________ Committee Member David A. Modarelli ii ABSTRACT This research was divided into two main parts. In the first part, a new facile route to relatively inexpensive thermoplastic polyphenoxyquinoxalines was developed. The synthetic route involves the aromatic nucleophilic substitution reaction of bisphenols with 2,3-dichloroquinoxaline. The dichloro monomer was prepared in two steps. In the first step, oxalic acid was condensed with o-phenylenediamine to give 2,3- dihydroxyquinoxaline. In the second step, 2,3-dihydroxyquinoxaline was treated with thionyl chloride to give 2,3-dichloroquinoxaline. This monomer was successfully polymerized with bisphenol-A, bisphenol-S, hexafluorobisphenol-A and 9,9-bis(4- hydroxyphenyl)fluorenone. Hydroquinone and biphenol, however, can not be polymerized to high molecular weight polymers because of the premature precipitation of crystalline oligomers. The glass transition temperatures of the high molecular weight polymers prepared from a series of bisphenols range from 191 °C to 279 °C, and their thermal decomposition temperatures are around 500 °C.