Handbook on Pesticidal Plants Optimization of Pesticidal Plants: Technology, Innovation, Outreach & Networks (Options)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vascular Plant Survey of Vwaza Marsh Wildlife Reserve, Malawi
YIKA-VWAZA TRUST RESEARCH STUDY REPORT N (2017/18) Vascular Plant Survey of Vwaza Marsh Wildlife Reserve, Malawi By Sopani Sichinga ([email protected]) September , 2019 ABSTRACT In 2018 – 19, a survey on vascular plants was conducted in Vwaza Marsh Wildlife Reserve. The reserve is located in the north-western Malawi, covering an area of about 986 km2. Based on this survey, a total of 461 species from 76 families were recorded (i.e. 454 Angiosperms and 7 Pteridophyta). Of the total species recorded, 19 are exotics (of which 4 are reported to be invasive) while 1 species is considered threatened. The most dominant families were Fabaceae (80 species representing 17. 4%), Poaceae (53 species representing 11.5%), Rubiaceae (27 species representing 5.9 %), and Euphorbiaceae (24 species representing 5.2%). The annotated checklist includes scientific names, habit, habitat types and IUCN Red List status and is presented in section 5. i ACKNOLEDGEMENTS First and foremost, let me thank the Nyika–Vwaza Trust (UK) for funding this work. Without their financial support, this work would have not been materialized. The Department of National Parks and Wildlife (DNPW) Malawi through its Regional Office (N) is also thanked for the logistical support and accommodation throughout the entire study. Special thanks are due to my supervisor - Mr. George Zwide Nxumayo for his invaluable guidance. Mr. Thom McShane should also be thanked in a special way for sharing me some information, and sending me some documents about Vwaza which have contributed a lot to the success of this work. I extend my sincere thanks to the Vwaza Research Unit team for their assistance, especially during the field work. -

PESTICIDAL PLANT LEAFLET Aloe Ferox Mill
PESTICIDAL PLANT LEAFLET Aloe ferox Mill. ROYAL BOTANIC GARDENS Taxonomy and nomenclature Family: Xanthorrhoeaceae (formerly Asphodelaceae) Synonym(s): Aloe candelabrum A. Berger (1906) Vernacular/ common names : (English): Red aloe, bitter aloe, cape aloe (French): Aloes du Cap Distribution and habitat A. ferox is indigenous to South Africa and Lesotho, growing in the semi-arid open plains to rocky mountain slopes. In Kenya it is commonly cultivated in Nairobi gardens and its environs. It is distributed throughout the tropics and sub tropics where it grows as an ornamental or medicinal plant. It grows in a wide range of climatic conditions, but abundant on arid, rocky hillsides up to 1000 mo altitude, where mean temperature ranges from Botanical description 27-31 C and annual rainfall is 50-300 mm. A. ferox is a single-stemmed plant growing up to 2-5 m tall. The crown is a dense rosette of green to red-brown succulent leaves up to 1 m long and the stem is covered Uses in persistent dried leaves. Each leaf has brown spines There are two main useful products obtained from A. along the margins and often on the surfaces. The flowers ferox. Aloe gel comes from the leaf parenchyma, the are bisexual, about 10 cylindrical racemes on a branched white inner fleshy part. It drains from the leaf when cut panicle, long with dark orange stamens protruding from and is used for its cleansing, antiseptic, moisturizing the mouth. Some forms can have bright red, yellow or and anti-inflamatory properties. Aloe bitters, the dark white flowers. sap comes from between the green peel and the white jelly and are used as a laxative and to treat arthritis. -

Toxicity in Artemia Salina by Hydroalcoholic Extracts Of
367 A publication of CHEMICAL ENGINEERING TRANSACTIONS VOL. 79, 2020 The Italian Association of Chemical Engineering Online at www.cetjournal.it Guest Editors: Enrico Bardone, Antonio Marzocchella, Marco Bravi Copyright © 2020, AIDIC Servizi S.r.l. DOI: 10.3303/CET2079062 ISBN 978-88-95608-77-8; ISSN 2283-9216 Toxicity in Artemia Salina by Hydroalcoholic Extracts of Monocotyledonous and Dicotyledonous Varieties of Medicinal Plants from the Peruvian Amazon Ana N. Sandoval*a, Jhonny W. Valverde Flores,b, Kriss M. Callaa, Rafael A. Albaa, a a c d Herry Lloclla , Santos A. Sotero Armando G. Ismiño , Marco L. Salazar aUniversidad César Vallejo, 22201, Cacatachi, San Martín, Perú. bUniversidad Nacional Agraria La Molina,15024, Lima, Perú. cAsociación de Productores Jardines de Palma, 22236, Pongo de Caynarachi, San Martin, Perú. d Universidad Nacional de Trujillo, 13006, Trujillo, Perú. [email protected] Medicinal plants have been used since ancient times, acquiring broad interest in their healing properties due to their active compounds. The Peruvian Amazon rainforest has a great variety of flora, such as monocots ( Dracontium loretense Krause and Commelina diffusa) and dicotyledons (Dysphania ambrosioides, Malva sylvestris, Origanum vulgare, Bixa orellana, Pinus edulis, Jatropha curcas L, and Brunfelsia). The study aimed to evaluate the toxicity in saline artemia by hydroalcoholic extracts of leaves of medicinal plants of the Peruvian Amazon. A phytochemical analysis of the leaves of the medicinal plants was performed to identify their active components. For the hydroalcoholic extraction, 300 g of leaves were used and dried extracts were obtained at concentrations of 10, 100 and 1000 μL/mL. The toxicity of the extract of each plant species in 10 larvae of Artemia was evaluated by triplicate tests. -

Mothers, Markets and Medicine Hanna Lindh
Mothers, markets and medicine The role of traditional herbal medicine in primary women and child health care in the Dar es Salaam region, Tanzania Hanna Lindh Degree project in biology, Bachelor of science, 2015 Examensarbete i biologi 15 hp till kandidatexamen, 2015 Biology Education Centre, Uppsala University Supervisors: Sarina Veldman and Hugo de Boer 1 Abstract Traditional medicine is still the most common primary healthcare used in Tanzania, especially among women. The ethnobotanical studies performed in Tanzania have not explored women’s traditional medicine, with the result that we do not know that much about it, including if women’s usage of medicinal plants create a threat against the medicinal flora’s biodiversity or not. Field studies consisting of interviews and collections of medicinal plants were carried out in the Dar es Salaam region in Tanzania before identifying the collected specimens by DNA barcoding, literature and morphology in Uppsala, Sweden. The 33 informants belonged to 15 different ethnic groups and 79% of them had migrated to Dar es Salaam. A total of 249 plant species were mentioned for women’s healthcare and 140 for children’s healthcare. The medicinal plants frequently reported as used for women’s health and childcare during structured interviews and free-listing exercises were Senna occidentalis/ Cassia abbreviata, Zanthoxylum sp., Clausena anisata, Acalypha ornata and Ximenia sp. The most salient uses of medicinal plants by women were during pregnancy, childbirth, menstruation, to induce abortion, and for cleansing infants and treating convulsions in children. Most of the fresh specimens were collected from disturbance vegetation. The informants having most interview answers in common were the market vendors, healers and herbalists and they were the only informants that mentioned species listed as vulnerable on the IUCN Red List of Threatened Species. -

Edible Flower and Herb and Pollinator Plant Varieties 2018
Edible Flower and Herb and Pollinator Plant Varieties 2018 Crop Type Variety Edible Flowers Alyssum Mixed Colors Edible Flowers Bachelor Buttons Polka Dot Edible Flowers Bellis English Daisy Strawberries and Cream Edible Flowers Bellis English Daisy Tasso Red Edible Flowers Borage Borago officinalis Borage Edible Flowers Calendula Solar Flashback Mix Edible Flowers Calendula Alpha Edible Flowers Calendula Resina Edible Flowers Calendula Neon Edible Flowers Calendula Triangle Flashback Edible Flowers Calendula Strawberry Blonde Edible Flowers Dianthus Everlast Lilac Eye Edible Flowers Dianthus Everlast Dark Pink Edible Flowers Dianthus Volcano Mix Edible Flowers Dianthus Dynasty Mix Edible Flowers Dianthus Everlast Edible Flowers Dianthus Pink Kisses Edible Flowers Marigold Lemon Gem Edible Flowers Marigold Tangerine Gem Edible Flowers Marigold Kilimanjaro White Edible Flowers Marigold Harlequin Edible Flowers Marigold Bonanza Deep Orange Edible Flowers Marigold Mister Majestic Edible Flowers Marigold Mexican Mint Edible Flowers Marigold Red Marietta Edible Flowers Marigold Bonanza Mix Edible Flowers Nasturtium Trailing Edible Flowers Nasturtium Alaska Edible Flowers Nasturtium Moonlight Edible Flowers Nasturtium Empress of India Edible Flowers Nasturtium Jewel Mix Edible Flowers Nigella Nigella damascena Perisan Jewels Edible Flowers Nigella Nigella sativa Black Cumin Edible Flowers Nigella Nigella hispanica Exotic Edible Flowers Sunflower Mammoth Edible Flower and Herb and Pollinator Plant Varieties 2018 Crop Type Variety Edible Flowers -

The Phytochemistry of Cherokee Aromatic Medicinal Plants
medicines Review The Phytochemistry of Cherokee Aromatic Medicinal Plants William N. Setzer 1,2 1 Department of Chemistry, University of Alabama in Huntsville, Huntsville, AL 35899, USA; [email protected]; Tel.: +1-256-824-6519 2 Aromatic Plant Research Center, 230 N 1200 E, Suite 102, Lehi, UT 84043, USA Received: 25 October 2018; Accepted: 8 November 2018; Published: 12 November 2018 Abstract: Background: Native Americans have had a rich ethnobotanical heritage for treating diseases, ailments, and injuries. Cherokee traditional medicine has provided numerous aromatic and medicinal plants that not only were used by the Cherokee people, but were also adopted for use by European settlers in North America. Methods: The aim of this review was to examine the Cherokee ethnobotanical literature and the published phytochemical investigations on Cherokee medicinal plants and to correlate phytochemical constituents with traditional uses and biological activities. Results: Several Cherokee medicinal plants are still in use today as herbal medicines, including, for example, yarrow (Achillea millefolium), black cohosh (Cimicifuga racemosa), American ginseng (Panax quinquefolius), and blue skullcap (Scutellaria lateriflora). This review presents a summary of the traditional uses, phytochemical constituents, and biological activities of Cherokee aromatic and medicinal plants. Conclusions: The list is not complete, however, as there is still much work needed in phytochemical investigation and pharmacological evaluation of many traditional herbal medicines. Keywords: Cherokee; Native American; traditional herbal medicine; chemical constituents; pharmacology 1. Introduction Natural products have been an important source of medicinal agents throughout history and modern medicine continues to rely on traditional knowledge for treatment of human maladies [1]. Traditional medicines such as Traditional Chinese Medicine [2], Ayurvedic [3], and medicinal plants from Latin America [4] have proven to be rich resources of biologically active compounds and potential new drugs. -

Fort Ord Natural Reserve Plant List
UCSC Fort Ord Natural Reserve Plants Below is the most recently updated plant list for UCSC Fort Ord Natural Reserve. * non-native taxon ? presence in question Listed Species Information: CNPS Listed - as designated by the California Rare Plant Ranks (formerly known as CNPS Lists). More information at http://www.cnps.org/cnps/rareplants/ranking.php Cal IPC Listed - an inventory that categorizes exotic and invasive plants as High, Moderate, or Limited, reflecting the level of each species' negative ecological impact in California. More information at http://www.cal-ipc.org More information about Federal and State threatened and endangered species listings can be found at https://www.fws.gov/endangered/ (US) and http://www.dfg.ca.gov/wildlife/nongame/ t_e_spp/ (CA). FAMILY NAME SCIENTIFIC NAME COMMON NAME LISTED Ferns AZOLLACEAE - Mosquito Fern American water fern, mosquito fern, Family Azolla filiculoides ? Mosquito fern, Pacific mosquitofern DENNSTAEDTIACEAE - Bracken Hairy brackenfern, Western bracken Family Pteridium aquilinum var. pubescens fern DRYOPTERIDACEAE - Shield or California wood fern, Coastal wood wood fern family Dryopteris arguta fern, Shield fern Common horsetail rush, Common horsetail, field horsetail, Field EQUISETACEAE - Horsetail Family Equisetum arvense horsetail Equisetum telmateia ssp. braunii Giant horse tail, Giant horsetail Pentagramma triangularis ssp. PTERIDACEAE - Brake Family triangularis Gold back fern Gymnosperms CUPRESSACEAE - Cypress Family Hesperocyparis macrocarpa Monterey cypress CNPS - 1B.2, Cal IPC -

A Review of Botany, Phytochemical, and Pharmacological Effects of Dysphania Ambrosioides
Indonesian Journal of Life Sciences Vol. 02 | Number 02 | September (2020) http://journal.i3l.ac.id/ojs/index.php/IJLS/ REVIEW ARTICLE A Review of Botany, Phytochemical, and Pharmacological Effects of Dysphania ambrosioides Lavisiony Gracius Hewis1, Giovanni Batista Christian Daeli1, Kenjiro Tanoto1, Carlos1, Agnes Anania Triavika Sahamastuti1* 1Pharmacy study program, Indonesia International Institute for Life-sciences, Jakarta, Indonesia *corresponding author. Email: [email protected] ABSTRACT Traditional medicine is widely used worldwide due to its benefits and healthier components that these natural herbs provide. Natural products are substances produced or retrieved from living organisms found in nature and often can exert biological or pharmacological activity, thus making them a potential alternative for synthetic drugs. Natural products, especially plant-derived products, have been known to possess many beneficial effects and are widely used for the treatment of various diseases and conditions. Dysphania ambrosioides is classified as an annual or short-lived perennial herb commonly found in Central and South America with a strong aroma and a hairy characteristic. Major components in this herb are ascaridole, p-cymene, α-terpinene, terpinolene, carvacrol, and trans-isoascaridole. Active compounds isolated from this herb are found to exert various pharmacological effects including schistosomicidal, nematicidal, antimalarial, antileishmanial, cytotoxic, antibacterial, antiviral, antifungal, antioxidant, anticancer, and antibiotic modulatory activity. This review summarizes the phytochemical compounds found in the Dysphania ambrosioides, together with their pharmacological and toxicological effects. Keywords: Dysphania ambrosioides; phytochemicals; pharmacological effect; secondary metabolites; toxicity INTRODUCTION pharmacologically-active compound, morphine, Natural products have been used by a wide was isolated from plants by Serturner spectrum of populations to alleviate and treat (Krishnamurti & Rao, 2016). -

Antibacterial Activity of Spent Substrate of Mushroom Pleurotus Ostreatus Enriched with Herbs
Journal of Agricultural Science; Vol. 7, No. 11; 2015 ISSN 1916-9752 E-ISSN 1916-9760 Published by Canadian Center of Science and Education Antibacterial Activity of Spent Substrate of Mushroom Pleurotus ostreatus Enriched with Herbs Maricela Ayala Martínez1, Deyanira Ojeda Ramírez1, Sergio Soto Simental1, Nallely Rivero Perez1, 2 1 Marcos Meneses Mayo & Armando Zepeda-Bastida 1 Área Académica de Medicina Veterinaria y Zootecnia, Instituto de Ciencias Agropecuarias, Universidad Autónoma del Estado de Hidalgo, México 2 Facultad de Ciencias de la Salud (Nutrición), Universidad Anáhuac México-Norte, México Correspondence: Armando Zepeda-Bastida, Área Académica de Medicina Veterinaria y Zootecnia, Instituto de Ciencias Agropecuarias, Universidad Autónoma del Estado de Hidalgo, Avenida Universidad s/n km 1, Tulancingo, Hidalgo, C.P. 43600, México. Tel: 52-771-717-2000 ext. 2449. E-mail: [email protected] Received: August 10, 2015 Accepted: September 11, 2015 Online Published: October 15, 2015 doi:10.5539/jas.v7n11p225 URL: http://dx.doi.org/10.5539/jas.v7n11p225 Abstract The recurrent use of antibiotics has given the guideline so that bacteria will develop resistance to drugs used in medicine, which is why recent investigations have been directed to evaluate natural sources such as plants or fungi, which can fight the bacteria. Here the antibacterial activity of spent substrate of Pleurotus ostreatus combined with medicinal plants was evaluated. We designed six mixtures (barley straw, barley straw/Chenopodium ambrosioides L., barley straw/Mentha piperita L., barley straw/Rosmarinus officinalis L., barley straw/Litsea glaucescens Kunth and barley straw/Tagetes lucid Cav) to be used as a substrate of cultivation of mushroom. -
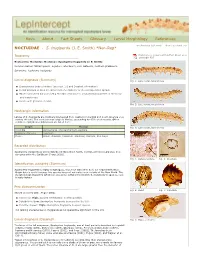
S. Frugiperda (J
Keys About Fact Sheets Glossary Larval Morphology References << Previous fact sheet Next fact sheet >> NOCTUIDAE - S. frugiperda (J. E. Smith) *Non-Rep* Taxonomy Click here to download this Fact Sheet as a printable PDF Noctuoidea: Noctuidae: Noctuinae: Spodoptera frugiperda (J. E. Smith) Common names: fall armyworm, cogollero, whorlworm, corn leafworm, southern grassworm Synonyms: Laphygma frugiperda Larval diagnosis (Summary) Fig. 1: Late instar, lateral view Characteristic body coloration (see Figs. 1-3 and Detailed Information) Dorsal pinacula of A1-8 are larger than the diameter of the corresponding spiracle Minute sclerotized bar connecting the SD1 setal base to a tonofibrillary platelet on the meso- and metathorax Cuticle with granulose texture Fig. 2: Late instar, lateral view Host/origin information Larvae of S. frugiperda are routinely intercepted from locations in Central and South America on a variety of hosts. The most common origin is Mexico, accounting for 65% of all records. Other common origin/host combinations are listed here: Origin Host(s) Fig. 3: Late instar, lateral view Colombia Alstroemeria, Chrysanthemum, Gerbera Dominican Republic Capsicum Mexico Apium, Brassica, Capsicum, Gladiolus, Ocimum, Zea mays Recorded distribution Spodoptera frugiperda is widely distributed throughout North, Central, and South America. It is also present in the Caribbean (Pogue 2002). Fig. 4: Cuticle texture Fig. 5: Crochets Identifcation authority (Summary) Spodoptera frugiperda is highly polyphagous, thus host data often does not help identification. Origin data is useful because this species does not naturally occur outside of the New World. The morphological characters listed here should be sufficient to identify S. frugiperda to species, even in early instars. -

Unusual Herbs
Unusual Herbs Fresh herbs add depth of flavor to food. We're all familiar with parsley, sage, rosemary and dill. But there are many less-common herbs you can grow to add some new flavor to your life. A favorite is Stevia rebaudiana. You are likely familiar with the sugar substitute Stevia, a refined version of this plant. The leaves alone are quite sweet and can be added to drinks and desserts to contribute sugar-free sweetness. Stevia is a perennial and will overwinter. It does not like soggy soil so make sure the soil drains well and dries out between watering. The leaves can be eaten fresh or dried for future use. For a decorative and edible addition to your garden, try the annual Perilla frutescens. Commonly known as shiso, it can be found in red and green varieties. The leaves are wide and attractive, somewhat resembling Coleus. P. frutescens grows well in full sun and likes moist, well-draining soil. It has a mild flavor and can be used raw as a garnish. Shiso has recently experienced a boost in popularity in the United States, and one can find dozens of recipes using it. For a bit more zing, try Dysphania ambrosioides, an annual known in the Mexican kitchen as epazote. It is native to Central America. Epazote is a leafy green with a flavor often described as pungent. It can be an acquired taste, but you may find the effort worthwhile as epazote is said to reduce flatulence caused by eating beans and some vegetables. The central valley has a prime climate for this herb. -

Chemical Composition, Antimicrobial and Antioxidant Properties of Seed Oil Plants of North-East India: a Review
Review Chemical composition, antimicrobial and antioxidant properties of seed oil plants of North-East India: A review Priyanka Saha1, Anupam Das Talukdar1*, Sanjoy Singh Ningthoujam1,2, Manabendra Dutta Choudhury1, Deepa Nath1,3, Lutfun Nahar4, Satyajit Dey Sarker4, Norazah Basar4,5 1Department of Life Science and Bioinformatics, Assam University, Silchar 788011, India; 2Department of Botany, Ghanapriya Women’s College, Imphal, Manipur, India; 3Department of Botany and Biotechnology, Karimganj College, Karimganj-788710. Assam India; 4Medicinal Chemistry and Natural Products Research Group, School of Pharmacy and Biomolecular Sciences, Liverpool John Moores University, Byrom Street, Liverpool L3 3AF, UK; 5Department of Chemistry, Faculty of Science, Universiti Teknologi Malaysia, 81310 Johor Bahru, Johor, Malaysia ABSTRACT Apart from being used as food, seed oils have also been used traditionally as medicinal products by several communities. However, the full medicinal potential of many seed oil plants is yet to be properly reviewed, particularly for their antimicrobial and antioxidant properties. North-East India has rich resources of seed oil plants. The availability of detailed information on these plants is quite limited. This review aims to explore and evaluate these seed oil plants of the North-East India with particular emphasis on their antimicrobial and antioxidant activities as well as chemical compositions. A comprehensive literature search on seed oil plants of this region has been performed. Seed oil yielding plants of this region can be categorized into two categories: plants that are used traditionally as sources of edible or medicinal oils and plants that are used for purposes other than as sources of oils. Many seed oil plants of this region have been reported to possess antimicrobial and antioxidant properties, and to produce various types of compounds.