Leptin in Anorexia Nervosa and Amenorrhea
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Corticotropin-Releasing Hormone Physiology
European Journal of Endocrinology (2006) 155 S71–S76 ISSN 0804-4643 Corticotropin-releasing hormone physiology Joseph A Majzoub Division of Endocrinology, Children’s Hospital Boston, Thomas Morgan Rotch Professor of Pediatrics, Harvard Medical School, 300 Longwood Avenue, Boston, Massachusetts 02115, USA (Correspondence should be addressed to J A Majzoub; Email: [email protected]) Abstract Corticotropin-releasing hormone (CRH), also known as corticotropin-releasing factor, is a highly conserved peptide hormone comprising 41 amino acid residues. Its name derives from its role in the anterior pituitary, where it mediates the release of corticotropin (ACTH) leading to the release of adrenocortical steroids. CRH is the major hypothalamic activator of the hypothalamic–pituitary– adrenal (HPA)axis. Major functions of the HPAinclude: (i) influencing fetal development of major organ systems including lung, liver, and gut, (ii) metabolic functions, including the maintenance of normal blood glucose levels during the fasting state via glycogenolysis and gluconeogenesis, (iii) modulation of immune function, and (iv) maintenance of cardiovascular tone. In addition, CRH, acting both directly and via the HPA, has a role in regulating several neuroendocrine functions including behavior, food intake, reproduction, growth, immune function, and autonomic function. CRH has been localized to the paraventricular nucleus (PVN) of the hypothalamus, which projects to the median eminence and other hypothalamic and midbrain targets. The CRH gene is composed of two exons. The CRH promoter contains a cAMP-response element, and the intron contains a restrictive element-1/neuron restrictive silencing element (RE-1/NRSE) sequence. Recently, a family of CRH-related peptides, termed the urocortins, has been identified. -

Associations Between Serum Leptin Level and Bone Turnover in Kidney Transplant Recipients
Associations between Serum Leptin Level and Bone Turnover in Kidney Transplant Recipients ʈ ʈ ʈ Csaba P. Kovesdy,*† Miklos Z. Molnar,‡§ Maria E. Czira, Anna Rudas, Akos Ujszaszi, Laszlo Rosivall,‡ Miklos Szathmari,¶ Adrian Covic,** Andras Keszei,†† Gabriella Beko,‡‡ ʈ Peter Lakatos,¶ Janos Kosa,¶ and Istvan Mucsi §§ *Division of Nephrology, Salem Veterans Affairs Medical Center, Salem, Virginia; †Division of Nephrology, University of Virginia, Charlottesville, Virginia; ‡Institute of Pathophysiology, Semmelweis University, Budapest, Hungary; §Harold Simmons Center for Chronic Disease Research & Epidemiology, Los Angeles Biomedical Research Institute at ʈ Harbor-University of California–Los Angeles Medical Center, Torrance, California; Institute of Behavioral Sciences, Semmelweis University, Budapest, Hungary; ¶First Department of Internal Medicine, Semmelweis University, Budapest, Hungary; **University of Medicine Gr T Popa, Iasi, Romania; ††Department of Epidemiology, Maastricht University, Maastricht, Netherlands; ‡‡Central Laboratory, Semmelweis University, Budapest, Hungary; and §§Division of Nephrology, Department of Medicine, McGill University Health Center, Montreal, Quebec, Canada Background and objectives: Obesity is associated with increased parathyroid hormone (PTH) in the general population and in patients with chronic kidney disease (CKD). A direct effect of adipose tissue on bone turnover through leptin production has been suggested, but such an association has not been explored in kidney transplant recipients. Design, setting, participants, & measurements: This study examined associations of serum leptin with PTH and with biomarkers of bone turnover (serum beta crosslaps [CTX, a marker of bone resorption] and osteocalcin [OC, a marker of bone formation]) in 978 kidney transplant recipients. Associations were examined in multivariable regression models. Path analyses were used to determine if the association of leptin with bone turnover is independent of PTH. -

Expression of Urocortin and Corticotropin-Releasing Hormone Receptors in the Horse Thyroid Gland
Cell Tissue Res DOI 10.1007/s00441-012-1450-4 REGULAR ARTICLE Expression of urocortin and corticotropin-releasing hormone receptors in the horse thyroid gland Caterina Squillacioti & Adriana De Luca & Sabrina Alì & Salvatore Paino & Giovanna Liguori & Nicola Mirabella Received: 11 January 2012 /Accepted: 3 May 2012 # Springer-Verlag 2012 Abstract Urocortin (UCN) is a 40-amino-acid peptide and UCN, CRHR1 and CRHR2 and that UCN plays a role in a member of the corticotropin-releasing hormone (CRH) the regulation of thyroid physiological functions through a family, which includes CRH, urotensin I, sauvagine, paracrine mechanism. UCN2 and UCN3. The biological actions of CRH family peptides are mediated via two types of G-protein-coupled Keywords Follicular cells . C-cells . RT-PCR . receptors, namely CRH type 1 receptor (CRHR1) and CRH Immunohistochemistry . Horse type 2 receptor (CRHR2). The biological effects of these peptides are mediated and modulated not only by CRH receptors but also via a highly conserved CRH-binding Introduction protein (CRHBP). Our aim was to investigate the expression of UCN, CRHR1, CRHR2 and CRHBP by immunohisto- Urocortin (UCN) is a peptide of 40 amino acids and is a chemistry, Western blot and reverse transcription with the member of the corticotropin-releasing hormone (CRH) fam- polymerase chain reaction (RT-PCR) in the horse thyroid ily, which includes CRH, urotensin I, sauvagine, UCN2 and gland. The results showed that UCN, CRHR1 and CRHR2 UCN3. Vaughan et al. (1995) were the first to identify UCN, were expressed in the thyroid gland, whereas CRHBP was which exhibits 45% homology to CRH (Latchman 2002; not expressed. -

Searching for Novel Peptide Hormones in the Human Genome Olivier Mirabeau
Searching for novel peptide hormones in the human genome Olivier Mirabeau To cite this version: Olivier Mirabeau. Searching for novel peptide hormones in the human genome. Life Sciences [q-bio]. Université Montpellier II - Sciences et Techniques du Languedoc, 2008. English. tel-00340710 HAL Id: tel-00340710 https://tel.archives-ouvertes.fr/tel-00340710 Submitted on 21 Nov 2008 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. UNIVERSITE MONTPELLIER II SCIENCES ET TECHNIQUES DU LANGUEDOC THESE pour obtenir le grade de DOCTEUR DE L'UNIVERSITE MONTPELLIER II Discipline : Biologie Informatique Ecole Doctorale : Sciences chimiques et biologiques pour la santé Formation doctorale : Biologie-Santé Recherche de nouvelles hormones peptidiques codées par le génome humain par Olivier Mirabeau présentée et soutenue publiquement le 30 janvier 2008 JURY M. Hubert Vaudry Rapporteur M. Jean-Philippe Vert Rapporteur Mme Nadia Rosenthal Examinatrice M. Jean Martinez Président M. Olivier Gascuel Directeur M. Cornelius Gross Examinateur Résumé Résumé Cette thèse porte sur la découverte de gènes humains non caractérisés codant pour des précurseurs à hormones peptidiques. Les hormones peptidiques (PH) ont un rôle important dans la plupart des processus physiologiques du corps humain. -
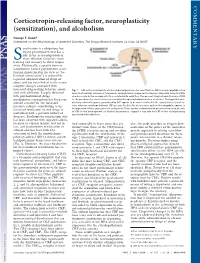
Corticotropin-Releasing Factor, Neuroplasticity (Sensitization), and Alcoholism
COMMENTARY Corticotropin-releasing factor, neuroplasticity (sensitization), and alcoholism George F. Koob* Committee on the Neurobiology of Addictive Disorders, The Scripps Research Institute, La Jolla, CA 92037 ensitization is a ubiquitous bio- logical phenomenon that has a role in the neuroadaptation of many different functions, from Slearning and memory to stress respon- sivity. Historically, a specific form of sensitization termed psychomotor sensi- tization (mistermed in my view as ‘‘be- havioral sensitization’’) is induced by repeated administration of drugs of abuse and has been linked to the neuro- adaptive changes associated with increased drug-seeking behavior associ- Fig. 1. CNS actions relevant to alcohol-induced psychomotor sensitization. CRF is a neuropeptide in the ated with addiction. Largely observed brain that controls autonomic, hormonal, and behavioral responses to stressors. New data show that CRF with psychostimulant drugs, also has a role in the neuroplasticity associated with addiction. The present study extends the role of CRF psychomotor sensitization has been con- to the psychomotor sensitization associated with repeated administration of alcohol. The hypothalamic- sidered a model for the increased pituitary-adrenal responses produced by CRF appear to be more involved in the acquisition of sensitiza- incentive salience contributing to the tion, whereas extrahypothalamic CRF systems, likely to be in structures such as the amygdala, appear to increased motivation to seek drugs in be important for the expression of sensitization. These results, combined with previous studies on the role of CRF in the development of alcohol dependence, suggest a key role for CRF in the neuroplasticity individuals with a previous history of associated with addiction. -

Urocortin 2 Modulates Glucose Utilization and Insulin Sensitivity in Skeletal Muscle
Urocortin 2 modulates glucose utilization and insulin sensitivity in skeletal muscle Alon Chen*†, Bhawanjit Brar*, Cheol Soo Choi‡, David Rousso*, Joan Vaughan*, Yael Kuperman†, Shee Ne Kim‡, Cindy Donaldson*, Sean M. Smith*, Pauline Jamieson*, Chien Li*, Tim R. Nagy§, Gerald I. Shulman‡, Kuo-Fen Lee*, and Wylie Vale*¶ *Clayton Foundation Laboratories for Peptide Biology, The Salk Institute for Biological Studies, La Jolla, CA 92037; †Department of Neurobiology, The Weizmann Institute of Science, Rehovot 76100, Israel; ‡Department of Internal Medicine and Cellular and Molecular Physiology and Howard Hughes Medical Institute, Yale University School of Medicine, New Haven, CT 06536; and §Department of Nutrition Sciences, University of Alabama at Birmingham, Birmingham, AL 35294 Contributed by Wylie Vale, August 28, 2006 Skeletal muscle is the principal tissue responsible for insulin- Results and Discussion stimulated glucose disposal and is a major site of peripheral insulin Generation of Ucn 2-Deficient Mice. To explore the physiological resistance. Urocortin 2 (Ucn 2), a member of the corticotropin- role of Ucn 2, we generated mice that are deficient in this releasing factor (CRF) family, and its cognate type 2 CRF receptor peptide. A genomic DNA clone containing Ucn 2 was isolated, (CRFR2) are highly expressed in skeletal muscle. To determine the and a targeting construct in which the full Ucn 2 coding sequence physiological role of Ucn 2, we generated mice that are deficient in was replaced with a neomycin-resistant gene cassette was gen- this peptide. Using glucose-tolerance tests (GTTs), insulin-tolerance erated (Fig. 1a). J1 ES cells were electroporated with the tests (ITTs), and hyperinsulinemic euglycemic glucose clamp stud- targeting construct and were selected as described in ref. -

Low Ambient Temperature Lowers Cholecystokinin and Leptin Plasma Concentrations in Adult Men Monika Pizon, Przemyslaw J
The Open Nutrition Journal, 2009, 3, 5-7 5 Open Access Low Ambient Temperature Lowers Cholecystokinin and Leptin Plasma Concentrations in Adult Men Monika Pizon, Przemyslaw J. Tomasik*, Krystyna Sztefko and Zdzislaw Szafran Department of Clinical Biochemistry, University Children`s Hospital, Krakow, Poland Abstract: Background: It is known that the low ambient temperature causes a considerable increase of appetite. The mechanisms underlying the changes of the amounts of the ingested food in relation to the environmental temperature has not been elucidated. The aim of this study was to investigate the effect of the short exposure to low ambient temperature on the plasma concentration of leptin and cholecystokinin. Methods: Sixteen healthy men, mean age 24.6 ± 3.5 years, BMI 22.3 ± 2.3 kg/m2, participated in the study. The concen- trations of plasma CCK and leptin were determined twice – before and after the 30 min. exposure to + 4 °C by using RIA kits. Results: The mean value of CCK concentration before the exposure to low ambient temperature was 1.1 pmol/l, and after the exposure 0.6 pmol/l (p<0.0005 in the paired t-test). The mean values of leptin before exposure (4.7 ± 1.54 μg/l) were also significantly lower than after the exposure (6.4 ± 1.7 μg/l; p<0.0005 in the paired t-test). However no significant cor- relation was found between CCK and leptin concentrations, both before and after exposure to low temperature. Conclusions: It has been known that a fall in the concentration of CCK elicits hunger and causes an increase in feeding activity. -

Leptin Replacement Reestablishes Brain Insulin Action in The
Diabetes Care 1 Sabine Frank-Podlech,1–3 Leptin Replacement Reestablishes Julia von Schnurbein,4 Ralf Veit,1–3 Martin Heni,2,3 Jurgen¨ Machann,2,5 Brain Insulin Action in the Jaana M. Heinze,2,3 Stephanie Kullmann,2,3 Jaida Manzoor,6 Hypothalamus in Congenital Saqib Mahmood,7 Hans-Ulrich Haring,¨ 2,3 fi Hubert Preissl,2,3,8,9 Martin Wabitsch,4 Leptin De ciency and Andreas Fritsche2,3 https://doi.org/10.2337/dc17-1867 OBJECTIVE Human obesity is associated with impaired central insulin signaling, and in very rare cases, severe obesity can be caused by congenital leptin deficiency. In such patients, leptin replacement results in substantial weight loss and improvement in peripheral 1 metabolism. Institute for Medical Psychology and Behaviou- ral Neurobiology, University of Tubingen,¨ Tubingen,¨ Germany RESEARCH DESIGN AND METHODS 2 Institute for Diabetes Research and Metabolic In a leptin-deficient patient, we investigated the impact of leptin substitution on Diseases of the Helmholtz Center Munich at the central insulin action, as quantified by changes in neuronal activity after intranasal University of Tubingen,¨ German Center for Dia- insulin application. This was assessed before and during the 1st year of metreleptin betes Research, Tubingen,¨ Germany 3Department of Internal Medicine IV, University substitution. Hospital, Tubingen,¨ Germany 4Division of Pediatric Endocrinology and Diabe- RESULTS tes, Department of Pediatrics and Adolescent After only 1 year, treatment with metreleptin reestablishes brain insulin sensitivity, Medicine, University of Ulm, Ulm, Germany 5 particularly in the hypothalamus and, to a lesser degree, in the prefrontal cortex. Section on Experimental Radiology, Depart- ment of Diagnostic and Interventional Radiol- Results are depicted in comparison with a control group. -

Metabolic Regulation of Fertility Through Presynaptic and Postsynaptic Signaling to Gonadotropin-Releasing Hormone Neurons
8578 • The Journal of Neuroscience, September 17, 2003 • 23(24):8578–8585 Behavioral/Systems/Cognitive Metabolic Regulation of Fertility through Presynaptic and Postsynaptic Signaling to Gonadotropin-Releasing Hormone Neurons Shannon D. Sullivan, R. Anthony DeFazio, and Suzanne M. Moenter 1Internal Medicine and Cell Biology, University of Virginia, Charlottesville, Virginia 22908 Gonadotropin-releasing hormone (GnRH) neurons form the final common pathway for the central regulation of reproduction and are inhibited by negative energy balance. In normal adults, these neurons maintain elevated intracellular chloride so that GABAA receptor activation is excitatory. We hypothesized that fasting alters homeostatic mechanisms to eliminate excitatory responses to GABA but rejected this hypothesis when brief, local GABA application elicited action currents in GnRH neurons from fed and fasted mice. This response was specific to GABAA receptors, because glycine elicited no response. We next found that fasting reduced the frequency of spontaneous GABAergic postsynaptic currents (PSCs) and that this was reversed by in vivo treatment with leptin during the fast. In the presence of tetrodotoxin to minimize presynaptic actions, leptin also potentiated the postsynaptic response of these cells to GABAA receptor activation. Postsynaptic effects of leptin on GABAergic miniature PSCs were eliminated by inhibiting JAK2/3 (Janus kinase), the tyrosine kinase through which leptin receptors signal. In all experiments, elimination of PSCs at ECl or by treatment with the GABAA receptor antagonist bicuculline confirmed that PSCs were specifically mediated by GABAA receptor chloride channels. These data dem- onstrate that fasting and leptin act presynaptically and postsynaptically to alter GABAergic drive to GnRH neurons, providing evidence for GABAergic communication of metabolic cues to GnRH neurons, and suggest the possibility for functional leptin receptors on GnRH neurons. -

Multi-Functionality of Proteins Involved in GPCR and G Protein Signaling: Making Sense of Structure–Function Continuum with In
Cellular and Molecular Life Sciences (2019) 76:4461–4492 https://doi.org/10.1007/s00018-019-03276-1 Cellular andMolecular Life Sciences REVIEW Multi‑functionality of proteins involved in GPCR and G protein signaling: making sense of structure–function continuum with intrinsic disorder‑based proteoforms Alexander V. Fonin1 · April L. Darling2 · Irina M. Kuznetsova1 · Konstantin K. Turoverov1,3 · Vladimir N. Uversky2,4 Received: 5 August 2019 / Revised: 5 August 2019 / Accepted: 12 August 2019 / Published online: 19 August 2019 © Springer Nature Switzerland AG 2019 Abstract GPCR–G protein signaling system recognizes a multitude of extracellular ligands and triggers a variety of intracellular signal- ing cascades in response. In humans, this system includes more than 800 various GPCRs and a large set of heterotrimeric G proteins. Complexity of this system goes far beyond a multitude of pair-wise ligand–GPCR and GPCR–G protein interactions. In fact, one GPCR can recognize more than one extracellular signal and interact with more than one G protein. Furthermore, one ligand can activate more than one GPCR, and multiple GPCRs can couple to the same G protein. This defnes an intricate multifunctionality of this important signaling system. Here, we show that the multifunctionality of GPCR–G protein system represents an illustrative example of the protein structure–function continuum, where structures of the involved proteins represent a complex mosaic of diferently folded regions (foldons, non-foldons, unfoldons, semi-foldons, and inducible foldons). The functionality of resulting highly dynamic conformational ensembles is fne-tuned by various post-translational modifcations and alternative splicing, and such ensembles can undergo dramatic changes at interaction with their specifc partners. -

Gut and Adipocyte Peptides
Reviews/Commentaries/ADA Statements PERSPECTIVES ON THE NEWS Gut and Adipocyte Peptides ZACHARY T. BLOOMGARDEN, MD sented information pertaining to effects of GLP-1 on insulin action, noting that a ma- jor confounding factor is the increase in his is the third in a series of articles els are increased. The perfused pancreas portal insulin levels and the decrease in Ϫ Ϫ on presentations at the American isolated from GLP-1R / animals shows glycemia with GLP-1, both indirectly im- T Diabetes Association Annual Meet- normal insulin response to glucose, proving the glucose-lowering effect of insu- ing, San Diego, California, 10–14 June although without response to GLP-1. GLP- lin. GLP-1 concentrations are somewhat Ϫ Ϫ 2005. 1R / -cells appear to have increased sen- lower in persons with diabetes and, to a sitivity to the incretin glucose-dependent lesser extent, with impaired glucose toler- Glucagon-like peptide-1 (and related insulinotropic polypeptide (GIP), perhaps ance, and GLP-1R activation is increased hormones) representing an adaptive upregulation of well beyond the physiologic range both Daniel Drucker (Toronto, Canada) re- GIP and/or of GIP response, and mice nei- with agonists and with administration of viewed evidence that the incretin effect, ther expressing the GLP-1 nor the GIP re- DPP-IV inhibitors. In vitro, GLP-1 increases the phenomenon of enteral glucose load- ceptor show glucose intolerance with adipocyte 2-deoxy-glucose uptake, rat so- ing increasing the insulin secretory re- decreased insulin response to glucose, and leus muscle glycogen synthesis, and hep- sponse to an increase in blood glucose, is fail to show a glucose-lowering response taocyte glycogen synthase A levels. -

NIDDK Recent Advances and Emerging Opportunities February
NIDDK: 60 Years of Discovery, 1950-2010 The NIDDK was established as the National Institute of Arthritis and Metabolic Diseases in 1950 by U.S. President Harry S. Truman. Sixty years and four name changes later, one thing has never changed: the Institute’s commitment to improving health through high-quality research. In 2010, NIDDK celebrated the rich history of advances made possible through the federal investment in research in our laboratories here in Bethesda, MD, and in Phoenix, AZ, and by grantees around the U.S. and beyond. NIDDK’S SCIENTIFIC SYMPOSIUM: CELEBRATES PAST, LOOKS TO FUTURE On Tuesday, September 21, 2010, NIDDK hosted, “Unlocking the Secrets of Science: Building the Foundation for Future Advances,” a scientific symposium to highlight research advances made possible, in part, with NIDDK support. NIDDK director, Dr. Griffin Rodgers, welcomed all in attendance to what promised to be an exciting day of cutting-edge scientific presentations. Former NIDDK directors Dr. Lester B. Salans (Mount Sinai Medical School and Forest Laboratories), Dr. Phillip Gorden (NIDDK), and Dr. Allen M. Spiegel (Albert Einstein College of Medicine) chaired scientific sessions on Diabetes and Digestive and Kidney Diseases; Nutrition, Hematology, and Urology; and Endocrinology, Education, Outreach, and the NIDDK Intramural Research Program. Each session featured three presentations from distinguished scientists spanning NIDDK’s research mission. Photo: Ernie Branson Session 1: Diabetes and Digestive and Kidney Diseases C. Ronald Kahn, M.D., Insulin