Optimization of Glipizide Floating Matrix Tablet Using Simplex Lattice Design
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Statement of Dividend Amount Transferred to IEPF
Statement of Dividend Transferred to IEPF_2019-20 Fno DpId Investor First Investor Investor Father Father Father Address State PIN Name Middle Last First Middle Last Name Name Name Name Name JAGANNA C/O S.N.BHOIR VASUDEV NIKETAN 1ST FLOOR- 80008624 MEDHA MAHESH KOLI MAHESH KOLI Maharashtra 421201 TH P.D.MARG DOMBIVALI (W) C/O. R.K. DUBEY E/3/34 JUNIOR M.I.G. ARERA 80010052 RANJANA DUBEY RAJESH KUMAR DUBEY Madhya Pradesh 462016 COLONY BHOPAL C/O MEHTA TRANDING COMPANY 10/1 80011593 HEMANT MEHTA MANAK CHANDJI MEHTA Madhya Pradesh 452007 BABADEEP COMPLEX MP MANAKCH C/O MEHTA TRANDING COMPANY 10/1 80011594 RAJESH MEHTA MEHTA Madhya Pradesh 452007 ANDJI BABADEEP COMPLEX MP AGRAWAL 104 JAWAHAR MARG BADNAWAR DIST 80011765 RAJESH AGRAWAL BABULAL JI Madhya Pradesh 454660 DHAR MP 80011978 SANDEEP LUCKY A N LUCKY 101, SAKET NAGAR INDORE M.P. Madhya Pradesh 452001 W/O COL RAJESH RANA B1/39 FIRST FLOOR 80012117 SUSHMA RANA CAPT RAJESH RANA Delhi 110058 JANAKPURI NEW Delhi HARBHAJ 80016434 JASPAL SINGH SALUJA SINGH SALUJA 39 TILAK MARG SONCTUCH DIST DEWAS (M.P.) Madhya Pradesh 455001 AN NARENDR MAHESH JAGANNATH KOLI C/O S.N.BHOIR 80008598 SAMIR BHOIR Maharashtra 421202 A VASUDEV DOMBIVALI (W) RAMCHAN RAMCHAN C/O. M/S. MAHADEV HARDWARE 2 MACHINERY 80010118 RAMESH KUMAR MOTIRAM Madhya Pradesh 460072 DANI DANI STORES MAIN BAIRAGARH SHRI NARESH CHANDRA HUMAD 14, SRINAGAR 80011183 SUNIL KUMAR JAIN G L JAIN Madhya Pradesh 450001 COLONY KHANDWA 80012360 LOKESH SAINI RAMESH CHANDRA SAINI SAINI BHAVAN NEAR RLY. STATION NEEMUCH Madhya Pradesh 458441 KHANDELW KHANDEL C/O KHANDELWAL MACHINERY STORE MAIN 80011868 SATISH RAJENDRA Madhya Pradesh 466001 AL WAL ROAD DIST. -

Appendix 8 Mandatory Disclosure Updated on 25.05.2018 1 AICTE
Appendix 8 Mandatory Disclosure Updated on 25.05.2018 1 AICTE file No. First: 06/01/GUJ/PHAR/2005/003 Current: Central/1-3515975459/2018/EOA Date & Time Period 30-6-2005 2 Name of the Institution Babaria Institute of Pharmacy Address of the Institution Vadodara-Mumbai NH # 8, City & Pin Code Varnama, Vadodara Pin-391240 State Gujarat Longitude & Latitude 22.189422 N & 73.186262 E Phone No. 0265-2303991 Fax No. 0265-2303999 Office Hours at the Institution 10.00 a.m. to 5.00 p.m. Academic hours at the 10.00 a.m. to 5.00 p.m. Institution Email [email protected] Website www.bitseducampus.org Nearest Railway Station(dist in Vadodara Railway Station (15 km) km) Nearest Airport (dist in km) Vadodara Airport (15 Km ) 3 Type of Institution Private- Self Financed Category (1) of the Institution Non Minority Category (2) of the Institution Co-Ed 4 Name of the organization Shree Krishna Educational Charitable Trust running the Institution Type of organization Charitable Trust Address of the organization 103, Gajanan Complex, Old Padra Road, Vadodara Registered with Asst. Charity Commissioner, Vadodara. E/5737/Vadodara Registration Date 13.10.2000 Website of the Organization www.bitseducampus.org 5 Name of the affiliating Gujarat Technological University University Address Nr.Vishwakarma Government Engineering College Nr.Visat Three Roads, Visat - Gandhinagar Highway Chandkheda, Ahmedabad – 382424 - Gujarat Phone: 079-23267521/570 Website www.gtu.ac.in Latest affiliation period Academic year 2018-19 6 Name of the Principal Dr. Vandana B. Patel Exact Designation Principal Phone Number with STD code 0265-2303991 Fax Number with STD code 0265-2303999 Email [email protected] Highest Degree Ph.D. -
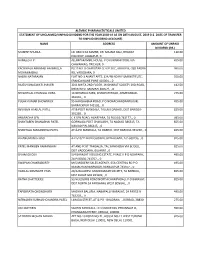
Name Address Amount of Unpaid Dividend (Rs.) Mukesh Shukla Lic Cbo‐3 Ka Samne, Dr
ALEMBIC PHARMACEUTICALS LIMITED STATEMENT OF UNCLAIMED/UNPAID DIVIDEND FOR THE YEAR 2018‐19 AS ON 28TH AUGUST, 2019 (I.E. DATE OF TRANSFER TO UNPAID DIVIDEND ACCOUNT) NAME ADDRESS AMOUNT OF UNPAID DIVIDEND (RS.) MUKESH SHUKLA LIC CBO‐3 KA SAMNE, DR. MAJAM GALI, BHAGAT 110.00 COLONEY, JABALPUR, 0 HAMEED A P . ALUMPARAMBIL HOUSE, P O KURANHIYOOR, VIA 495.00 CHAVAKKAD, TRICHUR, 0 KACHWALA ABBASALI HAJIMULLA PLOT NO. 8 CHAROTAR CO OP SOC, GROUP B, OLD PADRA 990.00 MOHMMADALI RD, VADODARA, 0 NALINI NATARAJAN FLAT NO‐1 ANANT APTS, 124/4B NEAR FILM INSTITUTE, 550.00 ERANDAWANE PUNE 410004, , 0 RAJESH BHAGWATI JHAVERI 30 B AMITA 2ND FLOOR, JAYBHARAT SOCIETY 3RD ROAD, 412.50 KHAR WEST MUMBAI 400521, , 0 SEVANTILAL CHUNILAL VORA 14 NIHARIKA PARK, KHANPUR ROAD, AHMEDABAD‐ 275.00 381001, , 0 PULAK KUMAR BHOWMICK 95 HARISHABHA ROAD, P O NONACHANDANPUKUR, 495.00 BARRACKPUR 743102, , 0 REVABEN HARILAL PATEL AT & POST MANDALA, TALUKA DABHOI, DIST BARODA‐ 825.00 391230, , 0 ANURADHA SEN C K SEN ROAD, AGARPARA, 24 PGS (N) 743177, , 0 495.00 SHANTABEN SHANABHAI PATEL GORWAGA POST CHAKLASHI, TA NADIAD 386315, TA 825.00 NADIAD PIN‐386315, , 0 SHANTILAL MAGANBHAI PATEL AT & PO MANDALA, TA DABHOI, DIST BARODA‐391230, , 0 825.00 B HANUMANTH RAO 4‐2‐510/11 BADI CHOWDI, HYDERABAD, A P‐500195, , 0 825.00 PATEL MANIBEN RAMANBHAI AT AND POST TANDALJA, TAL.SANKHEDA VIA BODELI, 825.00 DIST VADODARA, GUJARAT., 0 SIVAM GHOSH 5/4 BARASAT HOUSING ESTATE, PHASE‐II P O NOAPARA, 495.00 24‐PAGS(N) 743707, , 0 SWAPAN CHAKRABORTY M/S MODERN SALES AGENCY, 65A CENTRAL RD P O 495.00 -

TANVI THESIS-119997290053(1).Pdf
PHARMACOGNOSTIC, PHYTOCHEMICAL AND PSYCHOPHARMACOLOGICAL EVALUATION OF OLDENLANDIA CORYMBOSA AND GRANGEA MADERASPATANA A Thesis submitted to Gujarat Technological University for the Award of Doctor of Philosophy in Pharmacy By Tanvi D. Patel 119997290053 under supervision of Dr. Vineet C. Jain GUJARAT TECHNOLOGICAL UNIVERSITY AHMEDABAD January - 2018 © Tanvi D. Patel ii DECLARATION I declare that the thesis entitled Pharmacognostic, Phytochemical and Psychopharmacological evaluation of Oldenlandia corymbosa and Grangea maderaspatana submitted by me for the degree of Doctor of Philosophy is the record of research work carried out by me during the period from 2011 to 2018 under the supervision of Dr. Vineet Jain and this has not formed the basis for the award of any degree, diploma, associateship, fellowship, titles in this or any other University or other institution of higher learning. I further declare that the material obtained from other sources has been duly acknowledged in the thesis. I shall be solely responsible for any plagiarism or other irregularities, if noticed in the thesis. Signature of the Research Scholar : …………………………… Date:….……………… Name of Research Scholar: Tanvi D. Patel Place : Vadodara iii CERTIFICATE I certify that the work incorporated in the thesis Pharmacognostic, Phytochemical and Psychopharmacological evaluation of Oldenlandia corymbosa and Grangea maderaspatana submitted by Smt. Tanvi D. Patel was carried out by the candidate under my supervision/guidance. To the best of my knowledge: (i) the candidate has not submitted the same research work to any other institution for any degree/diploma, Associateship, Fellowship or other similar titles (ii) the thesis submitted is a record of original research work done by the Research Scholar during the period of study under my supervision, and (iii) the thesis represents independent research work on the part of the Research Scholar. -

Sno Dpid Folio/Clid Name Warrant No Total Shares Net Amount Micr No Bank Address-1 Address-2 Address-3 Address-4 Pincode
UJJIVAN FINANCIAL SERVICES LIMITED Dividend UNPAID REGISTER FOR THE YEAR INT. DIV. 2018-19 as on 31st December 2019 Sno Dpid Folio/Clid Name Warrant No Total_Shares Net Amount Micr No Bank Address-1 Address-2 Address-3 Address-4 Pincode 1 UFS0000238 Manali Thacker 400004 5886 5003.10 0 DDR 0 2 UFS0000252 Ratish.V.Rao 400005 3120 2652.00 0 DDR 0 3 120106 0500460060 KALAWATI . 400006 3650 3102.50 0 DDR 1/73, PROFESSOR COLONY, HANUMANGARH RAJASTHAN 335512 4 120177 0100528400 PUSHPA DEVI CHANDAK 400007 2300 1955.00 0 DDR BAHETI STREET POST NAGAUR . NAGAUR RAJASTHAN 341001 5 IN300513 15546439 JUGRAJ H JAIN 400008 4000 3400.00 0 DDR 1403 CENTRAL AVENUE DR D B MARG REALIENCE MALL MUMBAI CENTRAL MUMBAI MAHARASHTRA INDIA 400008 6 120475 0000001039 PRIYANKA MADHAVPRASAD AGARWAL 400009 6560 5576.00 0 DDR A 2 MATULYA CENTRE G R FLR SENAPATI BAPAT MARG LOWER PAREL MUMBAI MAHARASHTRA 400013 7 IN300214 14438623 SHYAMLAL AGARWAL 400011 3000 2550.00 0 DDR MADUBAN PLOT NO 59 5 INCOME TAX LANE 14 BHARATI NIWAS SOC PRABHAT ROAD PUNE 411004 8 IN300450 80534623 JADHAV SAMAR DILIP 400012 2026 1722.10 0 DDR BARAV BAUG LAXMINAGAR PHALTAN DIST SATARA PHALTAN 415523 9 160101 0000105177 DEEPAK NARAYAN SHINDE 400013 2530 2150.50 0 DDR 3 TARA APARTMENT OPP RAMAYAN TILAK WADI NASIK MAHARASHTRA 422002 10 IN300214 16344572 SUSHEELA GURUMUKH JAGHANI 400014 8720 7412.00 0 DDR PLOT NO 19, JAY NAGAR JALGAON MAHARASHTRA 425001 11 120113 0000001430 ANANTHAKRISHNAN SUBRAMANIAM IYER 400015 6206 5275.10 0 DDR PL NO. 105/1 106 PLAT NO. -

Black White.Cdr
I.M.A.G.S.B. NEWS BULLETIN AUGUST-2015 / MONTHLY NEWS Dr. Chetan N. Patel Vadodara (M) 94263 78078 Dr. Vinay A. Patel Dr. Bharat R. Patel Dr. Manhar A. Santwani Dr. Rashmi Upadhyay Dr. Sunil L. Acharya Dr. Pradeep Bhavsar Dr. Paresh P. Golwala Dr. Vinod Mehta Dr. Paresh Munshi Dr. Navin D. Patel Dr. S. S. Vaishya Dr. Bhaskar Mahajan Dr. Bhupendra M. Shah Himatnagar Dr. Bipin M. Patel Ahmedabad (15) I.M.A.G.S.B. NEWS BULLETIN AUGUST-2015 / MONTHLY NEWS I.M.A.G.S.B. NEWS BULLETIN AUGUST-2015 / MONTHLY NEWS decisions are not reaching to the grass root level workers because of communication gap between state branch, local branch & STATE PRESIDENT individual members. The data provided by you will help us to fill up AND this gap. Young members should be involved in all our activities. The HON. STATE SECRETARY'S wisdom of elders and vigor of youth are the key point for success to MESSAGE any organization. We should give more chances for the youth to take up the office and to run the office withDynamism. Every Dear Friends senior member should be a role model to the youthful members. Indian Medical Association is voluntary organization and is for Last year our respectable IMA Past National President Dr. Jitendra spreading and fostering brotherhood amongst ourselves. IMA is not B. Patel had launched two National project. IMA knowledge and only forum for Academic Interest, but also a forum to enhance the Life Style Disease Awareness programme. Our IMA GSB Past relationship between us. -

Interview List for Selection of Appointment of Notaries in the State of Gujarat
Interview List for Selection of Appointment of Notaries in the State of Gujarat Area of Practice S.No. Name File No. Father Name Address Enrollment no. Applied for 2, ManubhailS Chawl, Nisarahmed N- Ahmedabad Gulamrasul Near Patrewali Masjid G/370/1999 1 Gulamrasul 11013/2011/2016- Metropolitan City A.Samad Ansari Gomtipur Ahmedabad Dt.21.03.99 Ansari NC Gujarat380021 N- Gulamnabi At & Po.Anand, B, Nishant Ms. Merunisha G/1267/1999 2 Anand Distt. 11013/2012/2016- Chandbhai Colony, Bhalej Road, I Gulamnabi Vohra Dt.21.03.99 NC Vohra Anand Gujarat-388001 333, Kalpna Society, N- Deepakbhai B/H.Suryanagar Bus G/249/1981 3 Vadodara Distt. 11013/2013/2016- Bhikubhai Shah Bhikubhai Shah Stand, Waghodia Road, Dt.06.05.81 NC Vadodara Gujarat- N- Jinabhai Dhebar Faliya Kundishery Ms. Bakula G/267/1995 4 Junagadh 11013/2014/2016- Jesabhai Arunoday Junagadh Jinabhai Dayatar Dt.15.03.95 NC Dayatar Dist.Junagadh Gujarat- Mehta N- Vill. Durgapur, Tal. G/944/1999 5 Bharatkumar Mandvi-Kutch 11013/2015/2016- Hirji Ajramal Manvdi-Kutch Gujarat Dt.21.03.99 Hirji NC N- At.Kolavna, Ta.Amod, Patel Mohamedali G/857/1998 6 Bharuch Distt. 11013/2016/2016- Patel Yakub Vali Distt.Bharuch, Gujarat- Yakub Dt.09.10.98 NC 392140 N- Gandhi 6-B/1, Ajay Wadi, Gandhi Hitesh G/641/2000 7 Bhavnagar 11013/2017/2016- Vasantray Subhashnagar Bhavnagar Vasantray Dt.05.05.2000 NC Prabhudas Gujarat- 319, Suthar Faliyu, At. & N- Nileshkumar Jhagadiya, Dist. Motibhai PO Avidha, Ta. Jhagadiya, G/539/1995 8 11013/2018/2016- Motibhai Desai Bharuch Laxmidas Desai Dist. -

Ahmedabad Branch As on 27/08/2019 a B SHAH a D SHAH B-71, Kashivishveshwar Township, ‘Matru Prem’ 15, Ajantapark, Opp
Ahmedabad Branch as on 27/08/2019 A B SHAH A D SHAH B-71, Kashivishveshwar Township, ‘Matru Prem’ 15, AjantaPark, Opp. Bank of Baroda, Near Pizza AH-1 AH-2 B/H Ganesh Dugdhalaya, Bell, Jetalpur Road, Anand-388001 Vadodara 390007 (Gujarat) Email: [email protected] A K BHATT A M PATEL 50,Vaishnav Parivar, A/23, Jalaram Society, AH-3 Nr.Nehru Baug, Nr. Jiwan Deep AH-4 Nana Bazar, Society, Anand-388001 Vallabh Vidyanagar-388120 (Gujarat) (Gujarat) ASHOK KUMAR R PATEL B H DESAI 23/24, Avdhut Society, S D School of Commerce, AH-5 Opp Palika Nagar, AH-6 Gujarat University, Vallabh Vidyanagar-388120 Ahmedabad-380009 (Gujarat) (Gujarat) B N TRIVEDI B I PATEL 2, ChintanPark, ‘Panchvati’ Chitrakut Bunglows, N/B Mrudang Apt, Vasana, AH-7 B/h VaibhavTower, AH-8 Ahmedabad-380007 Vidyanagar Road, Anand-388001 (Gujarat) (Gujarat) BHARAT K OZA BHADRESH B GANDHI 1661-A Swati, Gokul (Vakil) Society, Tax Consultant, AH-9 AH-10 Sardarnagar, Amar Chambers, Station Road, Bhavnagar-364007 Valsad-396001 (Gujarat) (Gujarat) C V SOPARIWALA C K SONARA 11-B, Sardarnagar Society, D/66, University Colony, AH-11 AH-12 Tithal Road, Vallabh Vidhyanagar-388120 Valsad-396001 (Gujarat) (Gujarat) CHANDRAKANT R PATEL CHIRAG H JARIWALA 8, Abhishek Tenaments, Asopalav Bunglows, AH-13 D Z PatelHighSchool, AH-14 Shripal Nagar, OppKidneyHospital, Nr 80" Road, Anand-388001 Petlad Road, Nadiad-387001(Gujarat) (Gujarat) D A PATEL O-14, Akanxa Appartment, D M SHAH C/o B. K. Patel Flat, Nr. Sola 9-A, Govardhan Society, AH-15 AH-16 Railway Crossing, Sola Road, Opp APC, Anand Vidhyanagar -

RKLIEPF4 Web Upload.Xlsx
RADICO KHAITAN LIMITED IEPF 4 DATA ON DIVIDEND FOR THE FINANCIAL YEAR 201011 Nominal Value of Sr.No Folio No Name Address Pincode Shares Shares. 1 1201090002136489 SENU JOHN KOVOOR KOVOOR HOUSE CHIRAKADAVOM KAYAMKULAM KAYAMKULAM KERALA 690502 10 20.00 2 1201092400099691 LAXMAN PRASAD VILBHAGIPATTI KHURD POBHAGIPATTI JHEEL GOPALGANJ GOPALGANJ BIHAR 841438 25 50.00 RAMKISAN 3 1201320001533623 MOHANLAL KARWA 36, GANESH MARKET, DR.AMBEDKAR ROAD, NASIK ROAD NASIK MAHARASHTRA 422101 2 4.00 MITESH KANTIBHAI 4 1201860000090899 AHIR 1SAI ASHISH NAGAR SOC TADWADI RANDER ROAD SURAT GUJARAT 395009 50 100.00 5 1201860000260350 SALIL KUMAR SINGH NIKAT BARI MATHIA SATANI SARAI BALLIA UTTAR PRADESH 277001 100 200.00 6 1202920000099897 SHRIPATI YADAV 34/308,SECTOR3 PRATAP NAGAR SANGANER JAIPUR RAJASTHAN 302017 210 420.00 PERVEZ AHMAD 7 1202990003158196 QADRI 1116/1 SANGAM VIHAR COLONY SIPRI BAZAR JHANSI UTTAR PRADESH 284003 23 46.00 SANJAY ANANDRAO RS NO529, PLOT NO46, SARNAIK MAL, SAMRAT NAGAR, KOLHAPUR 8 1203500000191925 MANJREKAR MAHARASHTRA 416008 35 70.00 MEENA RAJENDRA 9 1203600001118736 KHANDAGALE 155 BUDHWAR PETH SATARA MAHARASHTRA 415002 10 20.00 SHEKH RASHID 10 1203840001059161 QURESHI AT42 SARKARI IMAMBADA BHIND MADHYAPRADESH 1 2.00 GOVABHAI NR MILK DAIRY AT AND PO GADH TAPALANPUR DIBANASKANTHA PALANPUR 11 1204470000224941 KUBERBHAI SALVI PALANPUR GUJARAT 385100 13 26.00 12 1204470001307884 KURIAN JOY KIZHAKKEPADAVATH HOUSE VELIMANAM PO IRITTY KANNUR KERALA 670673 81 162.00 SREEVANI VISWANATH RAO 13 1204470002489407 -

List of Trade Marks Agent As on 01/06/2017 Sr
List of Trade Marks Agent as on 01/06/2017 Sr. No. Name Address for communication Contact No. E-mail Certificate No. 1 MAULVI SAIFULLAH 3-5-139, Moti Voravad, Vijapur, Tal: Vijapur, Dist: 7926425258 [email protected] TMA/946 SHAMSUDDIN Meshana 382870, Gujarat 2 SHARAD ABHYANKAR 116/P, PANCHSHEEL-3, CO.0P. SOCIETY, RAHEJA 9820020790 [email protected] TMA/110 TOWNSHIP, MALAD(W), MUMBAI 3 DINESH MOHANAN NAIR C/O. R.K. DEWAN & CO., 3-A/13, RAMYA KUNJ, 9819422677 [email protected] TMA/100 GNPLOJEES CO.OP HSG. SOCIETY, P.O. JEKEGRAM, THANE - 400 606. 4 C. VENKATESAN #9 Andal Nagar Extension, Adambakkam, Chennai 600088 9444355395 [email protected] TMA/1000 5 KULDEEP SHARMA 265, A4, JANTA FLATS, PASCHIM VIHAR, NEW 880059089 TMA/1001 DELHI-110063. 6 ANUGU VIJAYA BHASKAR REDDY Plot No 8 and 9, 10th Cross, Celebrity Paradise Layout, 8861846598 [email protected] TMA/1002 Doddathoguru, Electronic City,Bangalore-560 100 7 JAY KIRANKUMAR JASANI RADHE ,13, Saurashtra Kala Kendra Society, Opp. Nirmala 8469988175 [email protected] TMA/1004 Convent School,RAJKOT - 360 005 (Gujarat). 8 BABU K.A. 1st FLOOR, RADHAGOPALAM NEAR GANAPATHI 9387289303 [email protected] TMA/1006 KOVIL, SANSKRIT COLLEGE ROAD, TRIPUNITHURA COCHIN - 682301 9 KUMARAN GOVINDASAMY A-203, MONT VERT BIARITZ-PHASE-II, OFF BANER- 9049800614 TMA/1007 PASHAN LINK ROAD PASHAN, PUNE - 411 021 10 RITEN MUNI A/74, VARMA VILLA, VITHALBHAI ROAD, VILE 9820344723 [email protected] TMA/1009 PARLE(WEST), MUMBAI-400056. 11 EASHWAR VENKATA 17/1,EASHWAR KUTEER, 8TH CROSS, 5TH MAIN, TMA/1011 SUBRAMANIAM N.R. -

Pharmainsights-2021-1
PHARMA INSIGHTS Triannual News Letter Jan - Feb - Mar - Apr 2021 Dear all, Seasons Greetings!! Here is the irst issue of the BIP newsletter for the year 2021. We hail the new year to bring new hopes, new aspirations for each one of you. The irst four months saw the students engaging themselves in various curricular and extracurricular activities for enhanced learning experience and wholistic development. Students and faculties have turned the pandemic into an opportunity rather than a roadblock. As the year 2021 takes shape and progresses further, let us strive to achieve higher milestones in our academic and extra curricular performance. Dr. Ajay K. Saluja Principal, Babaria Institute of Pharmacy 13/01/2021 LIVE session. The competition was judged by Dr. Falgun A. Mehta, Dr. Manisha Lalan and Dr. Meenakshi Patel. KITE FLYING FESTIVAL Evaluation of the Collage was based on the criteria like LIVE Collage Kite Making Competition creativity, originality, innovation, type of material used and Kite festival is one of the most popular festivals of India. It is theme covered. Based on the evaluation for Collage Kite also known as Makar Sankranti in most parts of India. The Making Competition, the entries from 1st Sem B. Pharm skyline of various cities across the state is illed with students, Vrushali Patel, Krena Patel and Vishwa Vyas were different sizes, varieties and colors of kites from before adjudged as the best 3 entries for the event. dawn until well after dark, presenting a spectacular view for the onlookers to behold. As to stop the spread of containment of COVID-19 amongst the people, no gathering and lying of kites at public places/open grounds/roads were allowed. -

Register of Accreditation of Fumigation Agencies for Aluminium Phosphide Fumigation (Nspm-22)
REGISTER OF ACCREDITATION OF FUMIGATION AGENCIES FOR ALUMINIUM PHOSPHIDE FUMIGATION (NSPM-22) Accrd. Name & Address of Accredited Telephone / E-mail ID Name Accredited Date of Issue Valid up to Redvalid No. Fumigation Agency FAX No. Fumigation Operator (s) (D/M/Y) (D/M/Y) ated up to (D/M/Y) / Remarks , if any. 001/Alp M/s L T Foods Ltd. Tel.: E-mail: Mr. Raj Deepak 25.11.2011 24.11.2012 Re- 43 Kms. Stone,G T Road, 0130-3051300 bhl.fumigation Accreditation Number: validated Bahalgarh, Sonepat- 131 021 Fax: @ltgroup.in 001011111 up to (Haryana) 0130-3051403 24/11/16 Mob: 09992114577 002/Alp M/s Jagatjit Industries Ltd. Ph.-0181 hamira@jagatjit Mr. Ajay Kumar 25.11.2011 24.11.2012 Re- P.O.-Jagatjit Nagar, Hamira, 2783112-16 .com Accreditation Number: validated District- Kapurthala Fax-0181 002011111 up to -144 802 (Punjab) 2783118 24/11/14 003/Alp M/s Jardine Henderson Mr. M. Prabhakar Reddy 25.11.2011 24.11.2012 Re- Limited, Accreditation Number: validated Shop No.-18, Gautam Complex, 003011111 up to Plot No. 17 & 18, Sector-11, 24/11/16 CBD Belapur, Navi Mumbai- Mr. Vinod Tiwari 400514 (Maharastra)* Accreditation Number: * Address Changed w.e.f. 003021111 14.09.15 Not renewed Mr. R. C. S. Ravi 08.01.2015 07.01.2016 Accreditation Number: 003030115 004/Alp M/s Star Agri-warehousing & Ph.-0141 www.staragri.c Mrs. Meenakshi Mangal 25.11.2011 24.11.2012 Re- Collateral Management Ltd. 3107465 om Accreditation Number: validated P. No. 29, G- 102, Molshree Fax-0141 004011111 up to Residency, Mission Compound, 2371079- 24/11/14 Ajmer Road, Jaipur – 302 006 Ext.29 Resigned from the (Rajasthan) agency Mr.