The Logic of Organic Synthesis Semester – Iv Cbcs System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Philosophy of Science and Philosophy of Chemistry
Philosophy of Science and Philosophy of Chemistry Jaap van Brakel Abstract: In this paper I assess the relation between philosophy of chemistry and (general) philosophy of science, focusing on those themes in the philoso- phy of chemistry that may bring about major revisions or extensions of cur- rent philosophy of science. Three themes can claim to make a unique contri- bution to philosophy of science: first, the variety of materials in the (natural and artificial) world; second, extending the world by making new stuff; and, third, specific features of the relations between chemistry and physics. Keywords : philosophy of science, philosophy of chemistry, interdiscourse relations, making stuff, variety of substances . 1. Introduction Chemistry is unique and distinguishes itself from all other sciences, with respect to three broad issues: • A (variety of) stuff perspective, requiring conceptual analysis of the notion of stuff or material (Sections 4 and 5). • A making stuff perspective: the transformation of stuff by chemical reaction or phase transition (Section 6). • The pivotal role of the relations between chemistry and physics in connection with the question how everything fits together (Section 7). All themes in the philosophy of chemistry can be classified in one of these three clusters or make contributions to general philosophy of science that, as yet , are not particularly different from similar contributions from other sci- ences (Section 3). I do not exclude the possibility of there being more than three clusters of philosophical issues unique to philosophy of chemistry, but I am not aware of any as yet. Moreover, highlighting the issues discussed in Sections 5-7 does not mean that issues reviewed in Section 3 are less im- portant in revising the philosophy of science. -

UMPOLUNG in REACTIONS CATALYZED by THIAMINE PYROPHOSPHATE DEPENDENT ENZYMES Umpolung En Reacciones Catalizadas Por Enzimas Dependientes De Pirofosfato De Tiamina
Ciencia, Ambiente y Clima, Vol. 2, No. 2, julio-diciembre, 2019 • ISSN (impreso): 2636-2317 • ISSN (en línea): 2636-2333 DOI: https://doi.org/10.22206/cac.2019.v2i2.pp27-42 UMPOLUNG IN REACTIONS CATALYZED BY THIAMINE PYROPHOSPHATE DEPENDENT ENZYMES Umpolung en reacciones catalizadas por enzimas dependientes de pirofosfato de tiamina Carlos José Boluda Emily Soto Instituto Tecnológico de Santo Domingo (INTEC), Instituto Tecnológico de Santo Domingo (INTEC), Área de Ciencias Básicas y Ambientales, Av. de Los Área de Ciencias Básicas y Ambientales Próceres 49, Santo Domingo, República Dominicana Correo-e: [email protected] *Corresponding author: Carlos J. Boluda Darah de la Cruz Correo-e: [email protected] Instituto Tecnológico de Santo Domingo (INTEC), Carolina Juncá Área de Ciencias Básicas y Ambientales Instituto Tecnológico de Santo Domingo (INTEC), Correo-e: [email protected] Área de Ciencias Básicas y Ambientales Anny Peña Correo-e: [email protected] Instituto Tecnológico de Santo Domingo (INTEC), Área de Ciencias Básicas y Ambientales Correo-e: [email protected] Recibido: 25/9/2019 • Aprobado: 19/10/2019 Cómo citar: Boluda, C. J., Juncá, C., Soto, E., de la Cruz, D., & Peña, A. (2019). Umpolung in reactions catalyzed by thiamine pyrophos- phate dependent enzymes. Ciencia, Ambiente Y Clima, 2(2), 27-42. Doi: https://doi.org/10.22206/cac.2019.v2i2.pp27-42 Abstract Resumen The temporal exchange of the electrophilic/nucleophilic El intercambio temporal del carácter electrofílico/nucleofí- character of an atom by chemical manipulation is known lico de un átomo mediante manipulación química, es cono- in organic chemistry as umpolung. This inversion of polarity cido con el vocablo alemán de umpolung. -

Catalytic Direct Asymmetric Michael Reactions
ORGANIC LETTERS 2001 Catalytic Direct Asymmetric Michael Vol. 3, No. 23 Reactions: Taming Naked Aldehyde 3737-3740 Donors Juan M. Betancort and Carlos F. Barbas III* The Skaggs Institute for Chemical Biology and the Department of Molecular Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, California 92037 [email protected] Received September 5, 2001 ABSTRACT Direct catalytic enantio- and diastereoselective Michael addition reactions of unmodified aldehydes to nitro olefins using (S)-2-(morpholinomethyl)- pyrrolidine as a catalyst are described. The reactions proceed in good yield (up to 96%) in a highly syn-selective manner (up to 98:2) with enantioselectivities approaching 80%. The resulting γ-formyl nitro compounds are readily converted to chiral, nonracemic 3,4-disubstituted pyrrolidines. The Michael reaction is generally regarded as one of the Typically, carbon nucleophiles that contain an active most efficient carbon-carbon bond forming reactions, and methylene center such as malonic acid esters, â-keto esters, studies concerning this reaction have played an important nitroalkanes, etc. have been studied in the Michael reaction. role in the development of modern synthetic organic Carbonyl compounds, and ketones in particular, have gener- chemistry.1 As the demand for optically active compounds ally only been used as donors following their preactivation has soared in recent years, much progress has been made in by conversion into a more reactive species such as enol or the development of asymmetric variants of this reaction, enamine equivalents.5,6 In these cases, additional synthetic providing for the preparation of Michael adducts with high enantiomeric purity.2 Though remarkable advances have been (3) (a) Chataigner, I.; Gennari, C.; Ongeri, S.; Piarulli, U.; Ceccarelli, S. -

Synthesis of Y,Δ-Unsaturated Amino Acids by Claisen Rearrangement - Last 25 Years
The Free Internet Journal Review for Organic Chemistry Archive for Arkivoc 2021, part ii, 0-0 Organic Chemistry to be inserted by editorial office Synthesis of y,δ-unsaturated amino acids by Claisen rearrangement - last 25 years Monika Bilska-Markowska,a Marcin Kaźmierczak,*a,b and Henryk Koroniaka aFaculty of Chemistry, Adam Mickiewicz University in Poznań, Uniwersytetu Poznańskiego 8, 61-614 Poznań, Poland bCentre for Advanced Technologies, Adam Mickiewicz University in Poznań, Uniwersytetu Poznańskiego 10, 61-614 Poznań, Poland Email: [email protected] In dedication to Professor Zbigniew Czarnocki on the occasion of his 66th anniversary Received mm-dd-yyyy Accepted mm-dd-yyyy Published on line mm-dd-yyyy Dates to be inserted by editorial office Abstract This mini review summarizes achievements in the synthesis of y,δ-unsaturated amino acids via Claisen rearrangements. The multitude of products that can be obtained using the discussed protocol shows that it is one of the most important reactions in organic synthesis. Moreover, many Claisen rearrangement products are building blocks in the synthesis of more complex molecules with potential biological activity. Keywords: y,δ-Unsaturated amino acids, Claisen rearrangement, fluorine-containing γ,δ-unsaturated amino acids, diastereoselectivity, optically active compounds DOI: https://doi.org/10.24820/ark.5550190.p011.335 Page 1 ©AUTHOR(S) Arkivoc 2021, ii, 0-0 Bilska-Markowska, M. et al. Table of Contents 1. Introduction 2. Chelated Claisen Rearrangement 3. Related Versions of Claisen Rearrangement for γ,δ-Unsaturated Amino Acids 4. Application of Claisen Rearrangement to the Synthesis of Fluorine-containing γ,δ-Unsaturated Amino Acids 5. -
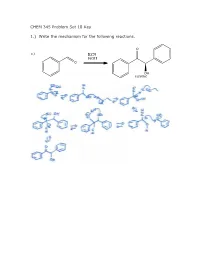
CHEM 345 Problem Set 18 Key 1.) Write the Mechanism for The
CHEM 345 Problem Set 18 Key 1.) Write the mechanism for the following reactions. O a.) KCN EtOH O OH racemic 1.) Write the mechanism for the following reactions. b.) KCN AcOH O NC OH racemic O c.) N S R O NEt3 OH racemic 2.) What is the structure of AcOH?Why does changing the solvent from EtOH to AcOH make such a big difference? O OH AcOH acetic acid The pKa of acetic acid is approximately 5. The pKa of ethanol is approximately 15. When you take a proton off of ethanol, you generate ethoxide which is about 1010 times stronger of a base than acetate. 3.) Give two instances when you need to use the thiazolium salt and triethylamine rather than KCN and EtOH. If the aldehydes contain an enolizable proton then you cannot use KCN/EtOH, instead you must use the thiazolium. Also, if the electrophile is a Michael acceptor to give a 1,4 dicarbonyl, then the thiazolium catalyst should be used. 4.) Break the following compound down as far as you can using Aldol, Michael, and Claisen reactions. Above each retrosynthetic arrow, write the name of the reaction. O HO O HO Aldol O Michael HO O O HO O Aldol O O Aldol HO O O HO Michael O O O Aldol There are other possibilities for order. O O HO Aldol O O 5.) Synthesize the following molecules. All carbons in the molecules must come from benzene or compounds with 5C’s or less. a.) O H2SO4 O HNO3 O2N AlCl3 O O SOCl2 HO Cl H2CrO4 1.) BuLi, Et2O + O 2.) H3O HO b.) O O O Cl + H3O NaOEt, EtOH O O O O Cl O AlCl3 Cl Cl AlCl3 Cl2 c.) O OMe NaOMe MeOH O O 1.) POCl3, DMF 2.) H2O OMe OMe O AlCl3 MeI Cl ONa HCl NaOH ZnHg 1.) NaOH + mcpba O 2.) H3O O OH O O AlCl3 Cl 6.) Write the mechanism for the following reactions. -

Peptide Chemistry up to Its Present State
Appendix In this Appendix biographical sketches are compiled of many scientists who have made notable contributions to the development of peptide chemistry up to its present state. We have tried to consider names mainly connected with important events during the earlier periods of peptide history, but could not include all authors mentioned in the text of this book. This is particularly true for the more recent decades when the number of peptide chemists and biologists increased to such an extent that their enumeration would have gone beyond the scope of this Appendix. 250 Appendix Plate 8. Emil Abderhalden (1877-1950), Photo Plate 9. S. Akabori Leopoldina, Halle J Plate 10. Ernst Bayer Plate 11. Karel Blaha (1926-1988) Appendix 251 Plate 12. Max Brenner Plate 13. Hans Brockmann (1903-1988) Plate 14. Victor Bruckner (1900- 1980) Plate 15. Pehr V. Edman (1916- 1977) 252 Appendix Plate 16. Lyman C. Craig (1906-1974) Plate 17. Vittorio Erspamer Plate 18. Joseph S. Fruton, Biochemist and Historian Appendix 253 Plate 19. Rolf Geiger (1923-1988) Plate 20. Wolfgang Konig Plate 21. Dorothy Hodgkins Plate. 22. Franz Hofmeister (1850-1922), (Fischer, biograph. Lexikon) 254 Appendix Plate 23. The picture shows the late Professor 1.E. Jorpes (r.j and Professor V. Mutt during their favorite pastime in the archipelago on the Baltic near Stockholm Plate 24. Ephraim Katchalski (Katzir) Plate 25. Abraham Patchornik Appendix 255 Plate 26. P.G. Katsoyannis Plate 27. George W. Kenner (1922-1978) Plate 28. Edger Lederer (1908- 1988) Plate 29. Hennann Leuchs (1879-1945) 256 Appendix Plate 30. Choh Hao Li (1913-1987) Plate 31. -

Ring Opening of Donor–Acceptor Cyclopropanes with N-Nucleo- Philes
SYNTHESIS0039-78811437-210X © Georg Thieme Verlag Stuttgart · New York 2017, 49, 3035–3068 short review 3035 en Syn thesis E. M. Budynina et al. Short Review Ring Opening of Donor–Acceptor Cyclopropanes with N-Nucleo- philes Ekaterina M. Budynina* Konstantin L. Ivanov Ivan D. Sorokin Mikhail Ya. Melnikov Lomonosov Moscow State University, Department of Chemistry, Leninskie gory 1-3, Moscow 119991, Russian Federation [email protected] Received: 06.02.2017 Accepted after revision: 07.04.2017 Published online: 18.05.2017 DOI: 10.1055/s-0036-1589021; Art ID: ss-2017-z0077-sr Abstract Ring opening of donor–acceptor cyclopropanes with various N-nucleophiles provides a simple approach to 1,3-functionalized com- pounds that are useful building blocks in organic synthesis, especially in assembling various N-heterocycles, including natural products. In this review, ring-opening reactions of donor–acceptor cyclopropanes with amines, amides, hydrazines, N-heterocycles, nitriles, and the azide ion are summarized. 1 Introduction 2 Ring Opening with Amines Ekaterina M. Budynina studied chemistry at Lomonosov Moscow 3 Ring Opening with Amines Accompanied by Secondary Processes State University (MSU) and received her Diploma in 2001 and Ph.D. in Involving the N-Center 2003. Since 2013, she has been a leading research scientist at Depart- 3.1 Reactions of Cyclopropane-1,1-diesters with Primary and Secondary ment of Chemistry MSU, focusing on the reactivity of activated cyclo- Amines propanes towards various nucleophilic agents, as well as in reactions -

Catalyzed Claisen Rearrangement of Allenyl Vinyl Ethers: a Synthetic and Mechanistic Approach Kassem M
Florida State University Libraries Electronic Theses, Treatises and Dissertations The Graduate School 2011 Gold (I)-Catalyzed Claisen Rearrangement of Allenyl Vinyl Ethers: A Synthetic and Mechanistic Approach Kassem M. Hallal Follow this and additional works at the FSU Digital Library. For more information, please contact [email protected] THE FLORIDA STATE UNIVERSITY COLLEGE OF ARTS AND SCIENCES GOLD (I)-CATALYZED CLAISEN REARRANGEMENT OF ALLENYL VINYL ETHERS; A SYNTHETIC AND MECHANISTIC APPROACH By KASSEM M. HALLAL A Dissertation submitted to the Department of Chemistry and Biochemistry in partial fulfillment of the requirements for the degree of Doctor of Philosophy Degree Awarded: Spring Semester, 2011 The members of the committee approve the dissertation of Kassem M. Hallal defended on March 18, 2011. _______________________________________ Marie E. Krafft Professor Directing Dissertation _______________________________________ Thomas C. S. Keller III University Representative _______________________________________ Robert A. Holton Committee Member _______________________________________ Gregory B. Dudley Committee Member _______________________________________ William T. Cooper Committee Member Approved: ____________________________________________________________ Joseph B. Schlenoff, Chair, Department of Chemistry and Biochemistry The Graduate School has verified and approved the above-named committee members. ii This work is dedicated To My soul mate, lovely wife zeinab, My baby Mohammad And also to my parents Mohammad & Kamela And to My brothers and lovely sister Youssef, Hamzeh and Fatima & My Great professor Prof. Marie E. Krafft iii ACKNOWLEDGEMENTS Starting my PhD career at Florida State University was one of the most important stages in my life. Throughout the past five years, I learned a lot of things about chemistry and science, however, the most important thing which I learned was the chemistry of life. -

A New Reaction Mechanism of Claisen Rearrangement Induced by Few-Optical-Cycle Pulses: Demonstration of Nonthermal Chemistry by Femtosecond Vibrational Spectroscopy*
Pure Appl. Chem., Vol. 85, No. 10, pp. 1991–2004, 2013. http://dx.doi.org/10.1351/PAC-CON-12-12-01 © 2013 IUPAC, Publication date (Web): 13 August 2013 A new reaction mechanism of Claisen rearrangement induced by few-optical-cycle pulses: Demonstration of nonthermal chemistry by femtosecond vibrational spectroscopy* Izumi Iwakura1,2,3,‡, Atsushi Yabushita4, Jun Liu2, Kotaro Okamura2, Satoko Kezuka5, and Takayoshi Kobayashi2 1Innovative Use of Light and Materials/Life, PRESTO, JST, 4-1-8 Honcho, Kawaguchi, Saitama, 332-0012, Japan; 2University of Electro-Communications, 1-5-1 Chofugaoka, Chofu, Tokyo 182-8585, Japan; 3Faculty of Engineering, Kanagawa University, 3-27-1 Rokkakubashi, Yokohama 221-8686, Japan; 4Department of Electrophysics, National Chiao-Tung University, Hsinchu 300, Taiwan; 5Department of Applied Chemistry, School of Engineering, Tokai University, 1117 Kitakaname, Hiratsuka-shi, Kanagawa 259-1292, Japan Abstract: Time-resolved vibration spectroscopy is the only known way to directly observe reaction processes. In this work, we measure time-resolved vibration spectra of the Claisen rearrangement triggered and observed by few-optical-cycle pulses. Changes in molecular structure during the reaction, including its transition states (TSs), are elucidated by observ- ing the transient changes of molecular vibration wavenumbers. We pump samples with visi- ble ultrashort pulses of shorter duration than the molecular vibration period, and with photon energies much lower than the minimum excitation energy of the sample. The results indicate that the “nonthermal Claisen rearrangement” can be triggered by visible few-optical-cycle pulses exciting molecular vibrations in the electronic ground state of the sample, which replaces the typical thermal Claisen rearrangement. -

Nuclear Magnetic Resonance Approaches in the Study of 2-Oxo Acid Dehydrogenase Multienzyme Complexes— a Literature Review
Molecules 2013, 18, 11873-11903; doi:10.3390/molecules181011873 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Review Nuclear Magnetic Resonance Approaches in the Study of 2-Oxo Acid Dehydrogenase Multienzyme Complexes— A Literature Review Sowmini Kumaran 1, Mulchand S. Patel 2 and Frank Jordan 1,* 1 Department of Chemistry, Rutgers University, Newark, NJ 07102, USA 2 Department of Biochemistry, School of Medicine and Biomedical Sciences, State University of New York at Buffalo, Buffalo, NY 14214, USA * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +1-973-353-5470; Fax: +1-973-353-1264. Received: 30 July 2013; in revised form: 14 September 2013 / Accepted: 16 September 2013 / Published: 26 September 2013 Abstract: The 2-oxoacid dehydrogenase complexes (ODHc) consist of multiple copies of three enzyme components: E1, a 2-oxoacid decarboxylase; E2, dihydrolipoyl acyl-transferase; and E3, dihydrolipoyl dehydrogenase, that together catalyze the oxidative decarboxylation of 2-oxoacids, in the presence of thiamin diphosphate (ThDP), coenzyme A 2+ + (CoA), Mg and NAD , to generate CO2, NADH and the corresponding acyl-CoA. The structural scaffold of the complex is provided by E2, with E1 and E3 bound around the periphery. The three principal members of the family are pyruvate dehydrogenase (PDHc), 2-oxoglutarate dehydrogenase (OGDHc) and branched-chain 2-oxo acid dehydrogenase (BCKDHc). In this review, we report application of NMR-based approaches to both mechanistic and structural issues concerning these complexes. These studies revealed the nature and reactivity of transient intermediates on the enzymatic pathway and provided site-specific information on the architecture and binding specificity of the domain interfaces using solubilized truncated domain constructs of the multi-domain E2 component in its interactions with the E1 and E3 components. -

Recoverable Phospha-Michael Additions Catalyzed by a 4-N,N
molecules Article Recoverable Phospha-Michael Additions Catalyzed by a 4-N,N-Dimethylaminopyridinium Saccharinate Salt or a Fluorous Long-Chained Pyridine: Two Types of Reusable Base Catalysts Eskedar Tessema 1,†, Vijayanath Elakkat 1,† , Chiao-Fan Chiu 2,3,*, Jing-Hung Zheng 1, Ka Long Chan 1, Chia-Rui Shen 4,5, Peng Zhang 6,* and Norman Lu 1,7,* 1 Institute of Organic and Polymeric Materials, National Taipei University of Technology, Taipei 106, Taiwan; [email protected] (E.T.); [email protected] (V.E.); [email protected] (J.-H.Z.); [email protected] (K.L.C.) 2 Department of Pediatrics, Linkou Medical Center, Chang Gung Memorial Hospital, Taoyuan 333, Taiwan 3 Graduate Institute of Clinical Medical Sciences, College of Medicine, Chang Gung University, Taoyuan 333, Taiwan 4 Department of Medical Biotechnology and Laboratory Sciences, College of Medicine, Chang Gung University, Taoyuan 333, Taiwan; [email protected] 5 Department of Ophthalmology, Linkou Medical Center, Chang Gung Memorial Hospital, Taoyuan 333, Taiwan 6 Department of Chemistry, University of Cincinnati, Cincinnati, OH 45221-0172, USA 7 Development Center for Smart Textile, National Taipei University of Technology, Taipei 106, Taiwan Citation: Tessema, E.; Elakkat, V.; * Correspondence: [email protected] (C.-F.C.); [email protected] (P.Z.); Chiu, C.-F.; Zheng, J.-H.; Chan, K.L.; [email protected] (N.L.) Shen, C.-R.; Zhang, P.; Lu, N. † These authors contributed equally to this work. Recoverable Phospha-Michael Additions Catalyzed by a Abstract: Phospha-Michael addition, which is the addition reaction of a phosphorus-based nucle- 4-N,N-Dimethylaminopyridinium ophile to an acceptor-substituted unsaturated bond, certainly represents one of the most versatile and Saccharinate Salt or a Fluorous powerful tools for the formation of P-C bonds, since many different electrophiles and P nucleophiles Long-Chained Pyridine: Two Types can be combined with each other. -

Lecture 1: Strategies and Tactics in Organic Synthesis
Massachusetts Institute of Technology Organic Chemistry 5.511 October 3, 2007 Prof. Rick L. Danheiser Lecture 1: Strategies and Tactics in Organic Synthesis Retrosynthetic Analysis The key to the design of efficient syntheses ". the grand thing is to be able to reason backwards. That is a very useful accomplishment, and a very easy "The end is where we start from...." one, but people do not practice it much." T. S. Eliot, in "The Four Quartets" Sherlock Holmes, in "A Study in Scarlet" Strategy Tactics overall plan to achieve the means by which plan ultimate synthetic target is implemented intellectual experimental retrosynthetic planning synthetic execution TRANSFORMS REACTIONS Target Precursor Precursor Target Definitions Retron Structural unit that signals the application of a particular strategy algorithm during retrosynthetic analysis. Transform Imaginary retrosynthetic operation transforming a target molecule into a precursor molecule in a manner such that bond(s) can be reformed (or cleaved) by known or reasonable synthetic reactions. Strategy Algorithm Step-by-step instructions for performing a retrosynthetic operation. "...even in the earliest stages of the process of simplification of a synthetic problem, the chemist must make use of a particular form of analysis which depends on the interplay between structural features that exist in the target molecule and the types of reactions or synthetic operations available from organic chemistry for the modification or assemblage of structural units. The synthetic chemist has learned by experience to recognize within a target molecule certain units which can be synthesized, modified, or joined by known or conceivable synthetic operations...it is convenient to have a term for such units; the term "synthon" is suggested.