Indian Injection Technique Study: Population Characteristics and Injection Practices
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Turks in India
t&55»^»m't:>;f.i; v5iw<iil^l««i^i«<%v.<w.4v THE TURKS IN INDIA CRITICAL CHAPTERS ON THE ADMINISTRATION OF THAT COUNTRY BY THE CHUGHTAI, BABAR, AND HIS DESCENDANTS BY HENRY GEORGE KEENE M.R.A.S. JUDGE OF AGRA FELLOW OF THE UNIVERSITY OF CALCUTTA AND AUTHOR OF " THE MOGHUL EMPIRE " LONDON W. H. ALLEN AND CO, 13 WATERLOO PLACE 1879 TO SIDNEY OWEN, M.A, IN ACKNOWLEDaMENT OF HIS VAltTABLE CONTEIBUTIONS TO OUE KNOWLEDGE OF INDIAN HISTORY 20G46:2 CONTENTS. CHAP. PAOE I. IXTEODUCTIOIT 1 II. The FoT7ifDATio]!f OF THE Chughtai Dynasty . 19 III. The Institutes of Akbas 52 IV. The Chaeactee of Jahangir .... 83 ' V. The Couet of Shahjahan- . 108 YI. India undee Afeungzeb 144 VII. Decay and Disintegeation 170 VIII. Decay and Disintegeation : II 200 IX. The Campaign of Panipat 228 Appendix 249 The object of the foliowing pages is to show, in a series of monographs, the character, epochs, and incidents in the history of the Empire established in Hindustan by the Chughtai Tartars. These chapters cover the time from the invasion of Babar to the death of Alamgir II., and the campaign of 1760-1. An attempt has been made to show the state of the country under Mughal rule, and the reasons why, with many good qualities, the House of Taimur ultimately failed to form a durable dominion. The first article is devoted to a summary of the subject of the whole study. The second gives a brief account of the origin of the family, and the first foundation of their power south of the Himala Alps. -
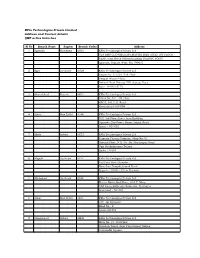
Kfin Technologies Private Limited Address and Contact Details QMF Active Branches
KFin Technologies Private Limited Address and Contact details QMF active branches Sl.No Branch Name Region Branch Codes Address 1 Agartala Guwahati AG01 KFIn Technologies Private Ltd. OLS RMS CHOWMUHANI,MANTRI BARI ROAD 1ST FLOOR NEAR Jana Sevak Saloon Building TRAFFIC POINT Agartala, Tripura West, Pin-799001 2 Agra Lucknow AG26 KFIn Technologies Private Ltd. House No. 17/2/4, 2nd Floor Deepak Wasan Plaza, Behind Hotel Holiday INN, Sanjay Place , Agra -282002 (U.P) 3 Ahmedabad Baroda AH01 KFIn Technologies Private Ltd. Office No. 401, 4th Floor ABC-I, Off. C.G. Road Ahmedabad-380 009 4 Ajmer New Delhi AJ44 KFIn Technologies Private Ltd. 302, 3rd Floor,Ajmer Auto Building Opposite City Power House,Jaipur Road Ajmer - 305 001 5 Akola Indore AK23 KFIn Technologies Private Ltd. Yamuna Tarang Complex, Shop No 30, Ground Floor, N.H. No- 06, Murtizapur Road Opp Radhakrishna Talkies Akola-444004 6 Aligarh Lucknow AL17 KFIn Technologies Private Ltd. 1st Floor Sevti Complex Near Jain Temple,Samad Road Aligarh - 202001, Uttar Pradesh 7 Allahabad Lucknow AL28 KFIn Technologies Private Ltd. Meena Bazar,2nd Floor, 10 S.P. Marg Civil Lines,Subhash Chauraha, Prayagraj Allahabad - 211001 8 Alwar New Delhi AL01 KFIn Technologies Private Ltd. 137, Jai Complex Road No - 2 Alwar-301001 9 Amaravathi Indore AM23 KFIn Technologies Private Ltd. Shop No. 21, 2nd Floor Gulshan Tower, Near Panchsheel Talkies, Jaistambh Square, Amaravathi - 444601 10 Ambala Lucknow AB40 KFIn Technologies Private Ltd. 6349,2nd Floor, Nicholson Road, Adjacent Kos Hospital, Ambala Cant, Ambala - 133001 11 Amritsar New Delhi AM41 KFIn Technologies Private Ltd. -

Chapter Ii History
CHAPTER II HISTORY ANCIENT PERIOD The archaeological discoveries prove that the region of Panipat was inhabited by human beings from very earlier times and had been the centre of vigorous cultural and political activity. We know from the hymns of the Rigveda (VII, 18,19; V.52,17) that the Bharatas of the Saraswati Valley held sway up to the Yamuna river and defeated Ajas, Sigrus and Yaksus1. The archaeological heritage of Panipat region can be divided into pre-historic, proto-historic and historical phases. The extent of archaeological sites of Panipat district, numbering 63, can be classified into Pre-Harappan, Harappan, Late Harappan, Painted Grey Ware (PGW), Grey Ware, Early Historical, Early Medieval and Medieval periods2. Alexander Cunningham and Rodgers were the first archaeologists who collected some relics specially coins from a few sites of Panipat. But it was in the year 1952 that B. B. Lal of Archaeological Survey of India discovered Painted Grey Ware and Northern Black Polished Ware from the mounds of Panipat and Sonepat. S. B. Chaudhary also discovered the Painted Grey Ware at Baholi, 13 kilometres to north-west of Panipat3. Subsequent archaeological explorations conducted by a number of archaeologists and recent explorations along the right bank of River Yamuna conducted under the supervision of C. Dorje have resulted to the discovery of Dull Red Ware in Garsh Sanrai in Panipat Tehsil and Red Ware, Red Polished Ware and Dull Red Ware in Jaurasi Khalsa in Samalkha Tehsil of Panipat district. These explorations have brought to light several ancient mounds containing the relics of bygone history4. -

Govt. of NCT of Delhi: Directorate of Education Examination Cell, Room No.222-A Old Secretariat, Delhi-110054
Govt. of NCT of Delhi: Directorate of Education Examination Cell, Room No.222-A Old Secretariat, Delhi-110054 No.DE.5/12/97/Exam/2013/ I 0 Date: U (10 11 (>22_1 Sub: Regarding All India Sainik Schools Entrance Exam- January 2021 This is for the information of all concerned that the date of Entrance Exam for AISSEE.2021-22 has been rescheduled for 07th Feb.2021(Sunday). This issues with reference to letter No.SSK/1302/AISSEE/2021-22 dated 07th December 2020 from the Principal, Sainik School Kunjpura, Karnal (Haryana) with the request to give wide publicity regarding aforesaid change of entrance exam date. Encl:As above ot • 0 (MUKTA SONI) DDE (EXAM) c") Phone : 0184-2384551 Sainik School Kunjpura Fax : 0184-2385059 Karnal (Haryana) Email [email protected] Pin-132023 No.SSK/1302/AISSEE/2021-22 s' • Dec 2020 The Chief Secretary Govt of Haryana Civil Secretariat CHANDIGARH The Chief Secretary Govt of Delhi 5, Sham Nadi' Marg, •1 • ') I ) 1, • • I DELHI — 110054 (.2 ) • Ivy( INTIMATION OF EXTENSION OF DATE OF ONLINE REGISTRATION AND CHANGE OF EXAM DATE OF ALL INDIA SANK SCHOOLS ENTRANCE EXAMINATION FOR THE ACADEMIC YEAR 2021-22 Sir, L!)\\T2 1. Please refer to this School letter of even number dated 29 Oct 2020 and 19 Nov 2020. 41/3 2. The last date for online registration of All India Sainik School Entrance Examination for -.L1‘\\ Y\ admission in Class VI & IX for the academic session 2021-22 has been extended till 18 Dec 202 The date of entrance exam has been rescheduled for 07 Feb 2921 (Stws10, The details are available on the NTA website hups://atssee.mamic.in and school We 'site www.ss:unjpura.org. -

1 Chapter One Kalpana Chawla Was Born in the Small Provincial City Of
Chapter One India Kalpana Chawla was born in the small provincial city of Karnal, Punjab (now Haryana), India, on 17 March 1962. Her birthdate was subsequently stated as 1 June 1961 to enable her to enroll early in school at her insistence. Her given name was “Montu”, and at age three, she chose “Kalpana”, meaning “imagination”, as her formal name. She was the youngest child of Banarsi lal (Pa-ji) (1) and Syongita (Chai-ji) Chawla, and she had two sisters, Sunita and Deepa, and one brother, Sanjay. Kalpana spent the first part of her childhood in what she always referred to as the “old house” in the Model Town area of Karnal. It was here that she lay on the roof and gazed into the night sky, pondering the mysteries of the constellations above. Pa-ji’s younger brother and his family lived at this house during Kalpana’s time there. She delighted in bossing around her younger cousins, Monnu and Jonnu, and making them stand in the hot sun, facing the garden wall, when they displeased her. In her early teenage years, Pa-ji moved his family into a large, comfortable house on Kunjpura Road, a stone’s throw from the Grand Trunk Road made famous by Rudyard Kipling in his book Kim. Kalpana rarely saw Pa-ji while she was growing up, as he spent long hours at his various business enterprises. Chai-ji spent much time attending to Pa-ji’s mother, who lived with the family, and her other children; by default, Sunita, the oldest sister, had a large role in raising the youngest member of the family, as is a common Punjabi custom. -

Composition and Role of the Nobility (1739-1761)
COMPOSITION AND ROLE OF THE NOBILITY (1739-1761) ABSTRACT OF THE THESIS SUBMITTED FOR THE AWARD OF THE DEGREE OF Boctor of ^l)iIo^op}|p IN HISTORY BY MD. SHAKIL AKHTAR Under the Supervision of DR. S. LIYAQAT H. MOINI (Reader) CENTRE OF ADVANCED STUDY DEPARTMENT OF HISTORY ALIGARH MUSLIM UNIVERSITY ALIGARH (INDIA) 2008 ABSTRACT Foregoing study ' composition and role of the Nobility (1739- 1761)', explore the importance of Nobility in Political, administrative, Sicio-cultural and economic spheres. Nobility , 'Arkan-i-Daullat' (Pillars of Empire) generally indicates'the'class of people, who were holding hi^h position and were the officers of the king as well as of the state. This ruling elite constituting of various ethnic group based on class, political sphere. In Indian sub-continent they served the empire/state most loyally and obediently specially under the Great Mughals. They not only helped in the expansion of the Empire by leading campaign and crushing revolt for the consolidation of the empire, but also made remarkable and laudable contribution in the smooth running of the state machinery and played key role in the development of social life and composite culture. Mughal Emperor Akbar had organized the nobility based on mgnsabdari system and kept a watchful eye over the various groups, by introducing local forces. He had tried to keep a check and balance over the activities, which was carried by his able successors till the death of Aurangzeb. During the period of Later Mughals over ambitious, self centered and greedy nobles, kept their interest above state and the king and had started to monopolies power and privileges under their own authority either at the court or in the far off provinces. -

KUNJEAN TIMES JANUARY‐2019 Sainik School Kunjpura, Karnal (Haryana)
DEEPAK KAPOOR KUNJEAN TIMES JANUARY‐2019 Sainik School Kunjpura, Karnal (Haryana) th INDEX 48 All India Sainik Schools Principals' Conference 2018 All India Sainik Schools Principals' Conference Inauguration of Conference Hall All India Sainik Schools Mountaineering Expedition 2018 Founder's Day 2018 Teachers' Day Celebration Inauguration of Motivational Hall 48th All India Sainik Schools Principals' 72nd Independence Day Conference was held at Sainik School Celebration Kunjpura, Karnal from 25th to 26th IH Athletics Competition October 2018. Dr Subhash Bhamre, Celebration of National Unity Hon'ble Raksha Rajya Mantri, Govt of Day India was the Chief Guest. Shri Manish Reunion of Golden Jubilee Thakur, Joint Secretary, Maj Gen KS Batch Nijjar, COS HQ 2 Corps & Chairman Old Boys Meet 2018 LBA, Dr Meenakshi Jolly, Director Trg, Installation of Open Air Gym Cmde G Rambabu, Gp Capt P Ravi Kumar Inspecting Officers and Principals of 27 Guest Lectures / Visits Sainik Schools attended the conference. After paying floral tribute to the martyrs at COC House Party Saikunj War Heroes Memorial, Dr Subhash Bhamre was given the Guard of Honour. Educational/ Motivational Besides delibration on various academic and administrative issues, the Chief Guest Tours also visited Motivation Hall, Atal Tinkering Lab and Artificial Climbing Wall. IPSC Corner Awards and Honours Inauguration of Conference Hall Activities At a Glance Sainik School Kunjpura is a Obituary trendsetter in developing unique infrastructure. The state of art EDITORIAL BOARD Conference Room was inaugurated Patron : Col VD Chandola by Commodore G Rambabu, Editor-in-Chief : Lt Col Astha Kotnala Inspecting Officer, Sainik Schools Editor : Sh VR Bhanot Sub-Editor : Smt RK Bajwa Society on 24 October 2018. -

PDF of Trial CTRI Website URL
PDF of Trial CTRI Website URL - http://ctri.nic.in Clinical Trial Details (PDF Generation Date :- Wed, 29 Sep 2021 20:06:07 GMT) CTRI Number CTRI/2012/07/002763 [Registered on: 04/07/2012] - Trial Registered Retrospectively Last Modified On 05/03/2020 Post Graduate Thesis No Type of Trial Observational Type of Study Cross Sectional Study Study Design Single Arm Trial Public Title of Study Prevalence of chronic kidney disease among Indian subjects with type-2 diabetes Scientific Title of A cross sectional observational study to determine the prevalence of chronic renal dysfunction Study among type 2 diabetes mellitus patients Secondary IDs if Any Secondary ID Identifier NIL NIL Details of Principal Details of Principal Investigator Investigator or overall Name Dr Sanjay Kalra Trial Coordinator (multi-center study) Designation Endocrinologist Affiliation Bharti Hospital Address Wazir Chand Colony, Kunjpura Road, Karnal, Haryana Karnal HARYANA 132001 India Phone 9896048555 Fax Email [email protected] Details Contact Details Contact Person (Scientific Query) Person (Scientific Name Dr Sanjay Kalra Query) Designation Endocrinologist Affiliation Bharti Hospital Address Wazir Chand Colony, Kunjpura Road, Karnal, Haryana Karnal HARYANA 132001 India Phone 9896048555 Fax Email [email protected] Details Contact Details Contact Person (Public Query) Person (Public Query) Name Dr Navneet Agarwal Designation Director Affiliation GR Medical College Address Diabetes, Obesity & Thyroid Center, 19, Hospital Road, Lalitpur Colony, Gwalior (M.P.) Gwalior MADHYA PRADESH 474001 India Phone 919826511222 Fax Email [email protected] page 1 / 4 PDF of Trial CTRI Website URL - http://ctri.nic.in Source of Monetary or Source of Monetary or Material Support Material Support > Shri Sitaram Shiksha evam Paryavaran Samaj Kalyan Samiti Reg Primary Sponsor Primary Sponsor Details Name Shri Sitaram Shiksha evam Paryavaran Samaj Kalyan Samiti Reg Address 2B/1, Yadav Colony, Near Ravishankar Medical Hostel, Gwalior, (M.P), India. -

Prospectus 2021-22
SAINIK SCHOOL KUNJPURA KARNAL(HARYANA) PROSPECTUS 2021-22 LAST DATE OF SUBMISSION OF ONLINE APPLICATION : 19 NOV 2020 DATE OF ENTRANCE EXAM : 10 JAN 2021 School Song Our School is a quiet and far away place, Where we're taught one and all to run life's race. We laugh and we sing, we weep a little too. But never never give in or rue. There's a grand grand school at Kunjpura, Where our hearts beat brave and true; Be the Thaneshwar or Kurukshetra, Chillianwala and Panipat too. In class or on the field we play the game. We seek not glamour nor hanker for fame; It's the fond desire and every boy's wish, To be always ready to accomplish There's a grand, grand school at Kunjpura Where our hearts beat brave and true; Be the Thaneshwar or Kurukshetra Chillianwala and Panipat too. 1 About us . Sainik School Kunjpura is one of the first five schools established by the Sainik Schools Society, New Delhi in 1961. At present there are 33 such schools covering all the states of the country. This school was founded by (late) Shri V.K. Krishna Menon, the then Defence Minister of India, on 24 July, 1961. The school is situated off the G.T. Road, 8 Kms away from Karnal Town. Aim The primary aim of the school is to prepare boys academically, mentally and physically for entry into the National Defence Academy. It enables boys from economically weaker sections of the society to take up an illustrious career as officers in the Armed Forces of the nation. -
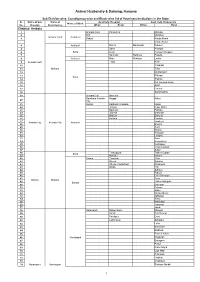
Sub Division, Block and Constituency Wise List Of
Animal Husbandry & Dairying, Haryana Sub Division-wise, Constituency-wise and Block-wise list of Veterinary Institutions in the State. Sr. Name of Sub Name of Govt.Vety. Hospital Govt. Vety. Dispensary Name of Block No. Division Constituency Urban Rural Urban Rural District: Ambala 1 Ambala Cantt Panjokhra Manglai 2 Boh Mandour Ambala Cantt Ambala II 3 Babyal Khuda Khurd Khuda Kalan 4 Ambala I Mohra Machonda Dukheri 5 Saha Khanpur 6 Saha Kesri Rampur Chappra 7 Samlehri Nanhera Pasiala 8 Ambala I Majri Shahpur Landa 9 Ambala Cantt Tepla Kesri 10 Chudiala 11 Mullana Toba 12 Mehtabgarh 13 Pilkhani Saha 14 Tharwa 15 Kot Kachwa Kalan 16 Ojlan 17 Durana 18 Sambhalkha 19 Ambala City Naneola Gaushala Ambala Naggal Kakru 20 City 21 Sonda Kathgarh Chappra Kaula 22 Jansua Rupa Majra 23 Baknour Panjola 24 Jalbera Batrohan 25 Mallour Baroula 26 Ballana Danipur Jandheri 27 Ambala City Ambala City Ambala I 28 Bhunni 29 Sonti 30 Khaira 31 Rasulpur 32 Lalyana 33 Bara 34 Bhanokheri 35 Kurbanpur 36 Chaurmastpur 37 Sullar Thakurpura Talheri Gujran 38 Saha 39 Mullana Nahoni 40 Barara Thambar Gola 41 Dheen Sohana 42 Sherpur Sulakhani Dhanoura 43 Ugala Holi 44 Adhoya 45 Rajouli 46 Dera Salimpur 47 Foxa Barara Mullana 48 Talheri Rangran Barara 49 Salehpur 50 Dulyani 51 Alipur 52 Manka Manki 53 Jaffarpur 54 Sehla 55 Kambassi 56 Tandwal 57 Jalubi 58 Naraingarh Mirpur Baroli Miyapur 59 Kurali Bari Rasour 60 Fatehpur Dera 61 Lakhnoura Akbarpur 62 Laha 63 Bari Kohri 64 Badhouli 65 Bhareri Kalan 66 Naraingarh Nagawan 67 Baragaon 68 Pinjori 69 Kathe Majra 70 Ujjal Majri 71 Pullewala 72 Gadholi 73 Chandsoli 74 Naraingarh Naraingarh Shahpur Nurdh 1 Animal Husbandry & Dairying, Haryana Sub Division-wise, Constituency-wise and Block-wise list of Veterinary Institutions in the State. -

(Kunjpura- Karnal-Kaithal-Khanauri Road). Besides, the Other Two St
west of Karnal , with which it is linked by a metalled road :' (Kunjpura- Karn al-Kaithal-Khanauri Ro ad) . Bes ide s, the other two State Highways whi ch pa ss through Kaithal are: (i) Kala Amb -Ambala-Pehowa-Kaithal-Nar- wana- Fat ehabad Road (S .H. 2) and (ii) Me erut -Sonepat -Gohana-A sandh- Kait hal-Pat iala Road (S.H . 11 ). It has al so a rai lway sta tion on the Kuruk- she tr a-Nar wan a section . Its popul ation was 34,8 90 in 196 1 an d in crea sed to 45, 199 in 1971 . Th e town is si tua te d on the bank s of an ex ten sive art ific ial lake , called the Bidki yar Lak e with numerou s bathing places and flights of steps. A high wa ll partl y of pakk a br ic ks and partly of mud en clo sed the town. It had 8 gates of whi ch Ka mal Gat e to th e eas t,Keor ak and Sur aj Kund Gate s to the north and Kas ai an d Dogran Gat@sto the west were the princip al ones. The se gates are now in a dil apid ated co ndition thou gh they still mark diff erent exits from the old town. Th e town is said to have be en founded by th e famou s Mahabharata hero, Yu dhisht hi ra, in commemoratio n of Pandava s' victory in Ma habh arata against th e Kauravas. -

Brief Industrial Profile of Karnal District
lR;eso t;rs Government of India Ministry of MSME Brief Industrial Profile of Karnal District Our Strength- gzekjh ’kfDr Carried out by:- MSME-Development Institute, Karnal (Ministry of MSME, Govt. of India) Phone: 0184 - 2230882 Fax: 0184 - 2231862 E-mail: [email protected] Web: www.msmedikarnal.gov.in [1] Contents S. No. Topic Page No. 1. General Characteristics of the District 3 1.1 Location & Geographical Area 3 1.2 Topography 3 1.3 Availability of Minerals. 4 1.4 Forest 4 1.5 Administrative set up 4 2. District at a glance 5 2.1 Existing Status of Industrial Area in the District Karnal 8 3. Industrial Scenario of District Karnal 9 3.1 Industry at a Glance 9 3.2 Year Wise Trend Of Units Registered 10 3.3 Details Of Existing Micro & Small Enterprises & Artisan Units In 11 The District 3.4 Large Scale Industries / Public Sector undertakings 12 3.5 Major Exportable Item 12 3.6 Growth Trend 12 3.7 Vendorisation / Ancillarisation of the Industry 12 3.8 Service Enterprises 12 3.8.1 Potential areas for service industry 12 3.9 Potential for new MSMEs 12 4. Existing Clusters of Micro & Small Enterprise 12 4.1 Details of Major Clusters 13 4.1.1 Manufacturing Sector 13 4.1.2 Service Sector 13 4.2 Details of Identified cluster 13 4.2.1 Karnal Pharmaceutical Cluster 13 4.2.2 Printing & Packaging Cluster 14 4.2.3 Karnal Paints & Chemicals Cluster 14 4.2.4 Agriculture Implements Cluster 15 5. General issues raised by industry association during the course 16 of meeting 6 Steps to set up MSMEs 17 7.