Biocatalytic Carbon Capture and Conversion
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Harnessing Escherichia Coli for Bio-Based Production of Formate
bioRxiv preprint doi: https://doi.org/10.1101/2021.01.06.425572; this version posted January 6, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Harnessing Escherichia coli for bio‐based production of formate under pressurized H2 and CO2 gases. Magali Roger1,2, Tom C. Reed2 and Frank Sargent2* 1 Aix Marseille University, CNRS, Bioenergetics and Protein Engineering (BIP UMR7281), 31 Chemin Joseph Aiguier, CS70071, 13042 Marseille Cedex 09, France. 2 School of Natural and Environmental Sciences, Newcastle University, Newcastle upon Tyne, NE1 7RU, England, UK *For Correspondence: Prof Frank Sargent FRSE, Division of Plant & Microbial Biology, School of Natural & Environmental Sciences, Newcastle University, Devonshire Building, Kensington Terrace, Newcastle upon Tyne NE2 4BF, England, United Kingdom. T: +44 191 20 85138. E: [email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/2021.01.06.425572; this version posted January 6, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. ABSRACT Escherichia coli is gram‐negative bacterium that is a workhorse of the biotechnology industry. The organism has a flexible metabolism and can perform a mixed‐acid fermentation under anaerobic conditions. Under these conditions E. coli synthesises a formate hydrogenlyase isoenzyme (FHL‐1) that can generate molecular hydrogen and carbon dioxide from formic acid. -

Medications in Pregnant and Nursing Mothers
Medications in Pregnant and Nursing Mothers NADINE M. GIRGIS, OD, FAAO ASSISTANT PROFESSOR YIN C. TEA, OD, FAAO CHIEF, PEDIATRICS AND BINOCULAR VISION ASSISTANT PROFESSOR Gestation age vs fetal age Gestation age-sperm penetrates the egg and zygote is formed Zygote (fertilized egg) travels from fallopian tube to uterus During this time, egg divides into cells - called a morula Continued dividing and morula - called a blastocyst - embeds in the uterus anywhere from 6-12 days after conception This begins the embryonic stage and fetal age begins Fetal development-1st trimester Gestation age week 3-fetal age week 1: a lot of basic growth Brain, spinal cord, heart, GI tract begin development 1st trimester Gestation age-week 4 and 5: embryo ¼ inch long Arm and leg buds, ears, eyes forming Placenta forming and producing hormones Heart is beating at a steady rhythm Movement of rudimentary blood through blood vessels 1st trimester Gestation age week 6: embryo is ½ in length Lungs, jaw, nose, plate formation, hands and feet Hand and feet buds have webbed structures Brain forming into complex parts 1st trimester Gestation age week 7: weighs less than an aspirin All essential organs have begun to form Hair, nail follicles, eyelids and tongue starting to form Trunk begins to straighten out 1st trimester Gestation age week 8: 1 in long, size of a bean All parts of adult are now present in the embryo Bones beginning to form Muscles begin to contract Facial features, including eyelids more developed Gestation age weeks 9-13: 3 in and weighs -

Recent Progress in the Microbial Production of Pyruvic Acid
fermentation Review Recent Progress in the Microbial Production of Pyruvic Acid Neda Maleki 1 and Mark A. Eiteman 2,* 1 Department of Food Science, Engineering and Technology, University of Tehran, Karaj 31587-77871, Iran; [email protected] 2 School of Chemical, Materials and Biomedical Engineering, University of Georgia, Athens, GA 30602, USA * Correspondence: [email protected]; Tel.: +1-706-542-0833 Academic Editor: Gunnar Lidén Received: 10 January 2017; Accepted: 6 February 2017; Published: 13 February 2017 Abstract: Pyruvic acid (pyruvate) is a cellular metabolite found at the biochemical junction of glycolysis and the tricarboxylic acid cycle. Pyruvate is used in food, cosmetics, pharmaceutical and agricultural applications. Microbial production of pyruvate from either yeast or bacteria relies on restricting the natural catabolism of pyruvate, while also limiting the accumulation of the numerous potential by-products. In this review we describe research to improve pyruvate formation which has targeted both strain development and process development. Strain development requires an understanding of carbohydrate metabolism and the many competing enzymes which use pyruvate as a substrate, and it often combines classical mutation/isolation approaches with modern metabolic engineering strategies. Process development requires an understanding of operational modes and their differing effects on microbial growth and product formation. Keywords: auxotrophy; Candida glabrata; Escherichia coli; fed-batch; metabolic engineering; pyruvate; pyruvate dehydrogenase 1. Introduction Pyruvic acid (pyruvate at neutral pH) is a three carbon oxo-monocarboxylic acid, also known as 2-oxopropanoic acid, 2-ketopropionic acid or acetylformic acid. Pyruvate is biochemically located at the end of glycolysis and entry into the tricarboxylic acid (TCA) cycle (Figure1). -

Fumarate Respiration of Wolinella Succinogenes: Enzymology, Energetics and Coupling Mechanism
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Biochimica et Biophysica Acta 1553 (2002) 23^38 www.bba-direct.com Review Fumarate respiration of Wolinella succinogenes: enzymology, energetics and coupling mechanism Achim Kro«ger a;*, Simone Biel a,Jo«rg Simon a, Roland Gross a, Gottfried Unden b, C. Roy D. Lancaster c a Institut fu«r Mikrobiologie, Johann Wolfgang Goethe-Universita«t, Marie-Curie-Str. 9, D-60439 Frankfurt am Main, Germany b Institut fu«r Mikrobiologie und Weinforschung, Johannes Gutenberg-Universita«t, D-55099 Mainz, Germany c Max-Planck-Institut fu«r Biophysik, Heinrich-Ho¡mann-Str. 7, D-60528 Frankfurt am Main, Germany Received 10 May 2001; received in revised form 27 August 2001; accepted 12 October 2001 Abstract Wolinella succinogenes performs oxidative phosphorylation with fumarate instead of O2 as terminal electron acceptor and H2 or formate as electron donors. Fumarate reduction by these donors (`fumarate respiration') is catalyzed by an electron transport chain in the bacterial membrane, and is coupled to the generation of an electrochemical proton potential (vp) across the bacterial membrane. The experimental evidence concerning the electron transport and its coupling to vp generation is reviewed in this article. The electron transport chain consists of fumarate reductase, menaquinone (MK) and either hydrogenase or formate dehydrogenase. Measurements indicate that the vp is generated exclusively by MK reduction with H2 or formate; MKH2 oxidation by fumarate appears to be an electroneutral process. However, evidence derived from the crystal structure of fumarate reductase suggests an electrogenic mechanism for the latter process. -

Preferred Drug List
October 2021 Preferred Drug List The Preferred Drug List, administered by CVS Caremark® on behalf of Siemens, is a guide within select therapeutic categories for clients, plan members and health care providers. Generics should be considered the first line of prescribing. If there is no generic available, there may be more than one brand-name medicine to treat a condition. These preferred brand-name medicines are listed to help identify products that are clinically appropriate and cost-effective. Generics listed in therapeutic categories are for representational purposes only. This is not an all-inclusive list. This list represents brand products in CAPS, branded generics in upper- and lowercase Italics, and generic products in lowercase italics. PLAN MEMBER HEALTH CARE PROVIDER Your benefit plan provides you with a prescription benefit program Your patient is covered under a prescription benefit plan administered administered by CVS Caremark. Ask your doctor to consider by CVS Caremark. As a way to help manage health care costs, prescribing, when medically appropriate, a preferred medicine from authorize generic substitution whenever possible. If you believe a this list. Take this list along when you or a covered family member brand-name product is necessary, consider prescribing a brand name sees a doctor. on this list. Please note: Please note: • Your specific prescription benefit plan design may not cover • Generics should be considered the first line of prescribing. certain products or categories, regardless of their appearance in • This drug list represents a summary of prescription coverage. It is this document. Products recently approved by the U.S. Food and not all-inclusive and does not guarantee coverage. -

Specific Pattern of Gene Expression During Induction of Mouse Erythroleukemia Cells
Downloaded from genesdev.cshlp.org on September 28, 2021 - Published by Cold Spring Harbor Laboratory Press Specific pattern of gene expression during induction of mouse erythroleukemia cells Peter J. Fraser and Peter J. Curtis The Wistar Institute of Anatomy and Biology, Philadelphia, Pennsylvania 19104 USA We have studied the expression of several characterized genes during induction of mouse erythroleukemia (MEL) cells with dimethyl sulfoxide (DMSO) and have observed a specific pattern of changes in transcriptional activity and steady-state RNA levels associated with erythroid differentiation. During induction there is a gradual, steady decrease in total transcriptional activity and RNA content per cell, which by day 3 of DMSO treatment amounts to less than 50% of the level in the uninduced cell. During this time we observe increases in transcriptional activity for 5-aminolevulinic acid synthase, carbonic anhydrase form II, and band 3 coordinate with the large increase in [3-globin gene transcription. The results also demonstrate an early decrease in transcription for carbonic anhydrase form I, which precedes decreases in transcription for glyceraldehyde phosphate dehydrogenase and rRNA genes. Changes in steady-state RNA levels reflected changes in transcriptional activity during induction except for carbonic anhydrase II mRNA. These results represent the first report characterizing the regulated expression at transcriptional and posttranscriptional levels of several known genes that are characteristically expressed in the erythrocyte. The results demonstrate that coordinate gene expression in erythroid differentiation occurs primarily at the level of transcription. [Key Words: Coordinated gene regulation; transcriptional activity; erythroid differentiation] Received June 25, 1987; revised version accepted August 14, 1987. Adult erythroid differentiation requires activation and/ dinately such that they may assemble to form the char- or modulation of many genes whose expression is regu- acteristic erythrocyte cytoskeleton. -
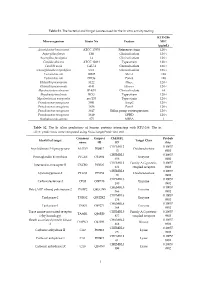
Table S1. the Bacterial and Fungal Isolates Used for the in Vitro Activity Testing
Table S1. The bacterial and fungal isolates used for the in vitro activity testing. KTU-286 Microorganism Strain No Feature MIC (µg/mL) Acinetobacter baummanii ATCC 17978 Reference strain 128 < Aspergillus flavus 12B Clinical isolate 128 < Aspergillus fumigatus 14 Clinical isolate 128 < Candida albicans ATCC 10231 Type strain 128 < Candida auris CAU-1 Clinical isolate 128 < Cuninghamella corymbifera CC1 Clinical isolate 128 < Escherichia coli 10025 Mcr-1 128 Escherichia coli DH5a Pan-S 128 Klebsiella pneumoniae 3122 blaKPC 128 < Klebsiella pneumoniae 4141 blaNDM-1 128 < Mycobacterium abscesus IP-K01 Clinical isolate 64 Mycobacterium bovis BCG Type strain 128 < Mycobacterium smegmatis mc2155 Type strain 128 < Pseudomonas aeruginosa 3691 AmpC 128 < Pseudomonas aeruginosa 3656 Pan-S 128 < Pseudomonas aeruginosa 3647 Efflux pump overexpression 128 < Pseudomonas aeruginosa 3619 OPRD 128 < Staphylococcus aureus 875 MRSA 1 Table S2. The In silico predictions of human proteins interacting with KTU-286. The in silico predictions were computed using SwissTargetPrediction tool. Common Uniprot ChEMBL Probab Identified target Target Class name ID ID ility CHEMBL2 0.10057 Arachidonate 5-lipoxygenase ALOX5 P09917 Oxidoreductase 15 8902 CHEMBL5 0.10057 Prostaglandin E synthase PTGES O14684 Enzyme 658 8902 CHEMBL2 Family A G protein- 0.10057 Interleukin-8 receptor B CXCR2 P25025 434 coupled receptor 8902 CHEMBL2 0.10057 Cyclooxygenase-2 PTGS2 P35354 Oxidoreductase 30 8902 CHEMBL3 0.10057 Carboxylesterase 2 CES2 O00748 Enzyme 180 8902 CHEMBL5 0.10057 Poly -

(12) United States Patent (10) Patent No.: US 7927,859 B2 San Et Al
USOO7927859B2 (12) United States Patent (10) Patent No.: US 7927,859 B2 San et al. (45) Date of Patent: Apr. 19, 2011 (54) HIGH MOLARSUCCINATEYIELD FOREIGN PATENT DOCUMENTS BACTERIA BY INCREASING THE WO WO99.06532 * 2/1999 INTRACELLULAR NADHAVAILABILITY WO WO 2007 OO1982 1, 2007 OTHER PUBLICATIONS (75) Inventors: Ka-Yiu San, Houston, TX (US); George N. Bennett, Houston, TX (US); Ailen Branden et al. Introduction to Protein Structure, Garland Publishing Inc., New York, p. 247, 1991.* Sánchez, Houston, TX (US) ExPASy. Formate Dehydrogenase.* Vemuri et al. Effects of growth mode and pyruvate carboxylase on (73) Assignee: Rice University, Houston, TX (US) Succinic acid production by metabolically engineered strains of Escherichia coli. Appl Environ Microbiol. Apr. 2002:68(4): 1715 (*) Notice: Subject to any disclaimer, the term of this 27.3 patent is extended or adjusted under 35 Goodbye et al. Cloning and sequence analysis of the fermentative alcohol-dehydrogenase-encoding gene of Escherichia coli. Gene. U.S.C. 154(b) by 791 days. Dec. 21, 1989;85(1):209-14.* Datsenko et al. One-step inactivation of chromosomal genes in (21) Appl. No.: 10/923,635 Escherichia coli K-12 using PCR products. Proc Natl AcadSci USA. Jun. 6, 2000:97(12):6640-5.* (22) Filed: Aug. 20, 2004 Berrios-Rivera et al. Metabolic engineering of Escherichia coli: increase of NADHavailability by overexpressing an NAD(+)-depen (65) Prior Publication Data dent formate dehydrogenase. Metab Eng. Jul. 2002;4(3):217-29.* Gupta et al. Escherichia coli derivatives lacking both alcohol US 2005/OO42736A1 Feb. 24, 2005 dehydrogenase and phosphotransacetylase grow anaerobically by lactate fermentation. -

Targeting Mycobacterium Tuberculosis Proteins: Structure and Function Studies of Five Essential Proteins
Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 411 Targeting Mycobacterium tuberculosis Proteins: Structure and Function Studies of Five Essential Proteins ADRIAN SUAREZ COVARRUBIAS ACTA UNIVERSITATIS UPSALIENSIS ISSN 1651-6214 UPPSALA ISBN 978-91-554-7134-7 2008 urn:nbn:se:uu:diva-8580 !! ""# !$"" % & % % '& &( )& * + &( ,- ( ""#( ) ' $ , , % + ' ( ( !!( .! ( ( /,0 12#31!34432!32( )& & & % - & &- % % 56 ( )& & % & % & % %% & ( )& % & 5 6 7!# 74## & (" !(2 8 ( 74## 7!# 93 & & * * %% % 3 - ( 7!14 & & *& && : & & (4 8 *&& % & & & ( & &; & %% ( < % & % & 5 6( )& & % - & * & % ( 7".. % ; * 3% & 93& 3 ' & *&& % &( 3 *& ( 44= % & ; * % ( 722# % & &3 & !(# 8 ( , & % & % & * ( &* ( & & & , // 7".. 7!# 7!14 74## 722# >3 & ! " # " #$ %&'" " ()*%+,- " ? ,- ""# /,,0 !.4!3.! /,0 12#31!34432!32 $ $$$ 3#4#" 5& $@@ (;(@ A B $ $$$ 3#4#"6 List of publications This thesis consists of a comprehensive summary based on the following papers. In the text, the papers will be referred to by their roman numerals. I Covarrubias, A. S., Larsson, A. M., Högbom, M., Lindberg, J., Bergfors, T., Björkelid, C., Mowbray, S. L., Unge, T., and Jones, T. A. (2005). Structure and -

NAD+-Dependent Formate Dehydrogenase from Plants
reVIeWS NAD+-dependent Formate Dehydrogenase from Plants A.A. Alekseeva1,2,3, S.S. Savin2,3, V.I. Tishkov1,2,3,* 1Chemistry Department, Lomonosov Moscow State University 2Innovations and High Technologies MSU Ltd 3Bach Institute of Biochemistry, Russian Academy of Sciences *E-mail: [email protected] Received 05.08.2011 Copyright © 2011 Park-media, Ltd. This is an open access article distributed under the Creative Commons Attribution License,which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT NAD+-dependent formate dehydrogenase (FDH, EC 1.2.1.2) widely occurs in nature. FDH consists of two identical subunits and contains neither prosthetic groups nor metal ions. This type of FDH was found in different microorganisms (including pathogenic ones), such as bacteria, yeasts, fungi, and plants. As opposed to microbiological FDHs functioning in cytoplasm, plant FDHs localize in mitochondria. Formate dehydrogenase activity was first discovered as early as in 1921 in plant; however, until the past decade FDHs from plants had been considerably less studied than the enzymes from microorganisms. This review summarizes the recent results on studying the physiological role, properties, structure, and protein engineering of plant formate dehy- drogenases. KEYWORDS plant formate dehydrogenase; physiological role; properties; structure; expression; Escherichia coli; protein engineering. ABBREVIATIONS FDH – formate dehydrogenase; PseFDH, CboFDH – formate dehydrogenases from -

The Green Synthesis and Molecular Docking of Novel N-Substituted
Bioorganic Chemistry 90 (2019) 103096 Contents lists available at ScienceDirect Bioorganic Chemistry journal homepage: www.elsevier.com/locate/bioorg The green synthesis and molecular docking of novel N-substituted T rhodanines as effective inhibitors for carbonic anhydrase and acetylcholinesterase enzymes ⁎ ⁎ Sinan Bayindira, , Cuneyt Caglayanb, Muhammet Karamanc, İlhami Gülcind, a Department of Chemistry, Faculty of Sciences and Arts, Bingol University, 12000-Bingöl, Turkey b Department of Biochemistry, Faculty of Veterinary Medicine, Bingol University, 12000-Bingöl, Turkey c Department of Molecular Biology and Genetics, Faculty of Arts and Science, Kilis 7 Aralik University, 79000-Kilis, Turkey d Department of Chemistry, Faculty of Sciences, Atatürk University, 25240-Erzurum, Turkey ARTICLE INFO ABSTRACT Keywords: Recently, inhibition effects of enzymes such as acetylcholinesterase (AChE) and carbonic anhydrase (CA)has Rhodanine appeared as a promising approach for pharmacological intervention in a variety of disorders such as epilepsy, Aza-ylides Alzheimer’s disease and obesity. For this purpose, novel N-substituted rhodanine derivatives (RhAs) were syn- Acetylcholinesterase thesized by a green synthetic approach over one-pot reaction. Following synthesis the novel compounds, RhAs Carbonic anhydrase derivatives were tested against AChE and cytosolic carbonic anhydrase I, and II (hCAs I, and II) isoforms. As a Enzyme inhibition result of this study, inhibition constant (Ki) were found in the range of 66.35 ± 8.35 to 141.92 ± 12.63 nM for Molecular docking AChE, 43.55 ± 14.20 to 89.44 ± 24.77 nM for hCA I, and 16.97 ± 1.42 to 64.57 ± 13.27 nM for hCA II, respectively. Binding energies were calculated with docking studies as −5.969, −5.981, and −9.121 kcal/mol for hCA I, hCA II, and AChE, respectively. -

Regulation of Pancreatic Islet Gene Expression in Mouse Islets by Pregnancy
265 Regulation of pancreatic islet gene expression in mouse islets by pregnancy B T Layden, V Durai, M V Newman, A M Marinelarena, C W Ahn, G Feng1, S Lin1, X Zhang2, D B Kaufman2, N Jafari3, G L Sørensen4 and W L Lowe Jr Division of Endocrinology, Metabolism and Molecular Medicine, Department of Medicine, Northwestern University Feinberg School of Medicine, 303 East Chicago Avenue, Tarry 15, Chicago, Illinois 60611, USA 1Northwestern University Biomedical Informatics Center, 2Division of Transplantation Surgery, Department of Surgery and 3Genomics Core, Center for Genetic Medicine, Northwestern University, Chicago, Illinois 60611, USA 4Medical Biotechnology Center, University of Southern Denmark, DK-5000 Odense C, Denmark (Correspondence should be addressed to W L Lowe Jr; Email: [email protected]) Abstract Pancreatic b cells adapt to pregnancy-induced insulin were confirmed in murine islets. Cytokine-induced resistance by unclear mechanisms. This study sought to expression of SP-D in islets was also demonstrated, suggesting identify genes involved in b cell adaptation during pregnancy. a possible role as an anti-inflammatory molecule. Comple- To examine changes in global RNA expression during menting these studies, an expression array was performed to pregnancy, murine islets were isolated at a time point of define pregnancy-induced changes in expression of GPCRs increased b cell proliferation (E13.5), and RNA levels were that are known to impact islet cell function and proliferation. determined by two different assays (global gene expression This assay, the results of which were confirmed using real- array and G-protein-coupled receptor (GPCR) array). time reverse transcription-PCR assays, demonstrated that free Follow-up studies confirmed the findings for select genes.