Suppression of Oriental Fruit Moth {Grapholita Molesta, Lepidoptera: Tortricidae) Populations Using the Sterile Insect Technique
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

FT Grapholita Pro Caps
M2i technology Unique patented process of pheromone micro-encapsulation Controled rate of pheromone release for greater efficiency 100% biodegradable Easy storage, at room temperature Extended shelf life: 2,5 years User guide M2i recommends: Grapholita Pro Caps® syringe + Delta trap © M2i Trap setup: empty the content of the syringe into the cup. Remove the protective film from the sticky sheet. Stick the cup containing the pheromone formulation in the middle of the sheet. Place the sticky sheet in the trap. The moths are attracted by the sexual pheromone, enter the trap and are caught. Characteristics of Grapholita Pro Caps® Type of product Pheromone dispenser Use Monitoring Z8-dodecenyl acetate, E8-dodecenyl acetate, Active substance © M2i Z8-dodecenol Volume of formulation 1,2 mL Indicative diffusion* 3 months Targeted insect life-stage Adult (moth) Estimated radius of diffusion Moths attracted on a radius of 5-10 m * depending on climatic conditions, for an average temperature of 30°C and without strong winds. Monitoring setup Detection period: from March to October (adapt and renew the pheromone dispenser according to the recommended diffusion time). Trap location: hung on the upper part of the tree’s canopy. © M2i Recommended density: 1-2 traps/ha Pest monitoring and recommendations Trap follow-up frequency Weekly Recommended intervention 8 moths/trap/week During the critical season and depending on trapping levels: it is possible to perform an additional Pest control methods insecticide and/or a biocontrol treatment according to the insect life stage. Refer to recommendations of registered products for plant protection (ephy.anses.fr) and/or to your technical advisor. -

Distinction of Grapholita Molesta Busck and Grapholita Dimorpha Komai Larvae Based on Morphological Feature of Anal Prolegs
九州大学学術情報リポジトリ Kyushu University Institutional Repository Distinction of Grapholita molesta Busck and Grapholita dimorpha Komai larvae based on morphological feature of anal prolegs Lee, Seung–Yeol College of Agriculture and Life Sciences, Kyungpook National University Choi, Kwang–Shik College of Natural Sciences, Kyungpook National University Back, Chang–Gi National Institute of Horticultural and Herbal Science, Rural Development Administration Choi, Kyung–Hee National Institute of Horticultural and Herbal Science, Rural Development Administration 他 https://doi.org/10.5109/1526340 出版情報:九州大学大学院農学研究院紀要. 60 (2), pp.291-295, 2015-09-18. 九州大学大学院農学研究 院 バージョン: 権利関係: J. Fac. Agr., Kyushu Univ., 60 (2), 291–295 (2015) Distinction of Grapholita molesta Busck and Grapholita dimorpha Komai larvae based on morphological feature of anal prolegs Seung–Yeol LEE1, Kwang–Shik CHOI2, Chang–Gi BACK3, Kyung–Hee CHOI3, In–Kyu KANG1, Hee–Young JUNG1* and Shoji OHGA* Laboratory of Forest Resources Management, Division of Forest Environmental Sciences, Department of Agro–environmental Sciences, Faculty of Agriculture, Kyushu University, Sasaguri, Fukuoka 811–2415, Japan (Received April 14, 2015 and accepted May 19, 2015) Larvae of Grapholita molesta Busck and Grapholita dimorpha Komai, which are major moth pests that affect apples in Korea, are very difficult to identify because of their morphological similarities. In this study, we investigated how to distinguish the larvae of these two species by using specific morphological features. Between 2013 and 2014, a total of 84 specimens were collected from apples suspected of infestation in Gunwi–gun and Cheongsong–gun, Gyeongsangbuk–do, Korea, and they were observed using a stereo microscope, optical microscope, and scanning electron microscope. -

Thaumatotibia Leucotreta
Thaumatotibia leucotreta Scientific Name Thaumatotibia leucotreta (Meyrick) Synonyms: Cryptophlebia leucotreta (Meyrick), Cryptophlebia roerigii Zacher Olethreutes leucotreta Meyrick Thaumatotibia roerigii Zacher Common Name(s) False codling moth, citrus codling moth, orange moth, and orange codling moth Type of Pest Moth Figure 1. Larva of Thaumatotibia leucotreta (T. Grove Taxonomic Position and W. Styn, bugwood.org). Class: Insecta, Order: Lepidoptera, Family: Tortricidae Reason for Inclusion CAPS Target: AHP Prioritized Pest List - 2003 through 2014 Pest Description Eggs: Eggs are flat, oval (0.77 mm long by 0.60 mm wide) shaped discs with a granulated surface. The eggs are white to cream colored when initially laid. They change to a reddish color before the black head capsule of the larvae becomes visible under the chorion prior to hatching (Daiber, 1979a). 1 Larvae: First instar (neonate) larvae approximately 1 to 1.2 mm (< /16 in) in length with dark pinacula giving a spotted appearance, fifth instar larvae are orangey-pink, 1 becoming more pale on sides and yellow in ventral region, 12 to 18 mm (approx. /2 to 11 /16 in) long, with a brown head capsule and prothoracic shield (Fig. 1). [Note this coloration is only present in live specimens.] The last abdominal segment bears an anal comb with two to ten “teeth.” The mean head capsule width for the first through fifth instar larvae has been recorded as: 0.22, 0.37, 0.61, 0.94 and 1.37 mm, respectively (Daiber, 1979b). Diagnostic characters would include the anal comb with two to ten teeth in addition to: L pinaculum on T1 enlarged and extending beneath and beyond (posterad of) the spiracle; spiracle on A8 displaced posterad of SD pinaculum; crochets unevenly triordinal, 36-42; L-group on A9 usually trisetose (all setae usually on same pinaulum) (Brown, 2011). -

Small Ermine Moths Role of Pheromones in Reproductive Isolation and Speciation
CHAPTER THIRTEEN Small Ermine Moths Role of Pheromones in Reproductive Isolation and Speciation MARJORIE A. LIÉNARD and CHRISTER LÖFSTEDT INTRODUCTION Role of antagonists as enhancers of reproductive isolation and interspecific interactions THE EVOLUTION TOWARDS SPECIALIZED HOST-PLANT ASSOCIATIONS SUMMARY: AN EMERGING MODEL SYSTEM IN RESEARCH ON THE ROLE OF SEX PHEROMONES IN SPECIATION—TOWARD A NEW SEX PHEROMONES AND OTHER ECOLOGICAL FACTORS “SMALL ERMINE MOTH PROJECT”? INVOLVED IN REPRODUCTIVE ISOLATION Overcoming the system limitations Overview of sex-pheromone composition Possible areas of future study Temporal and behavioral niches contributing to species separation ACKNOWLEDGMENTS PHEROMONE BIOSYNTHESIS AND MODULATION REFERENCES CITED OF BLEND RATIOS MALE PHYSIOLOGICAL AND BEHAVIORAL RESPONSE Detection of pheromone and plant compounds Introduction onomic investigations were based on examination of adult morphological characters (e.g., wing-spot size and color, geni- Small ermine moths belong to the genus Yponomeuta (Ypo- talia) (Martouret 1966), which did not allow conclusive dis- nomeutidae) that comprises about 75 species distributed glob- crimination of all species, leading to recognition of the so- ally but mainly in the Palearctic region (Gershenson and called padellus-species complex (Friese 1960) which later Ulenberg 1998). These moths are a useful model to decipher proved to include five species (Wiegand 1962; Herrebout et al. the process of speciation, in particular the importance of eco- 1975; Povel 1984). logical adaptation driven by host-plant shifts and the utiliza- In the 1970s, “the small ermine moth project” was initiated tion of species-specific pheromone mating-signals as prezy- to include research on many aspects of the small ermine gotic reproductive isolating mechanisms. -

Phylogeny of Tortricidae (Lepidoptera): a Morphological Approach with Enhanced Whole
Template B v3.0 (beta): Created by J. Nail 06/2015 Phylogeny of Tortricidae (Lepidoptera): A morphological approach with enhanced whole mount staining techniques By TITLE PAGE Christi M. Jaeger AThesis Submitted to the Faculty of Mississippi State University in Partial Fulfillment of the Requirements for the Degree of Master of Science in Agriculture and Life Sciences (Entomology) in the Department of Biochemistry, Molecular Biology, Entomology, & Plant Pathology Mississippi State, Mississippi August 2017 Copyright by COPYRIGHT PAGE Christi M. Jaeger 2017 Phylogeny of Tortricidae (Lepidoptera): A morphological approach with enhanced whole mount staining techniques By APPROVAL PAGE Christi M. Jaeger Approved: ___________________________________ Richard L. Brown (Major Professor) ___________________________________ Gerald T. Baker (Committee Member) ___________________________________ Diana C. Outlaw (Committee Member) ___________________________________ Jerome Goddard (Committee Member) ___________________________________ Kenneth O. Willeford (Graduate Coordinator) ___________________________________ George M. Hopper Dean College of Agriculture and Life Sciences Name: Christi M. Jaeger ABSTRACT Date of Degree: August 11, 2017 Institution: Mississippi State University Major Field: Agriculture and Life Sciences (Entomology) Major Professor: Dr. Richard L. Brown Title of Study: Phylogeny of Tortricidae (Lepidoptera): A morphological approach with enhanced whole mount staining techniques Pages in Study 117 Candidate for Degree of Master of -

Tortricoidea: Lepidoptera) from Northwestern India -- Tribe Eucosmini (Olethreutinae)
PAPER ZOOS' PRINT JOURNAL 20(2): 1751-1765 TAXONOMIC STUDIES ON THE FAMILY TORTRICIDAE (TORTRICOIDEA: LEPIDOPTERA) FROM NORTHWESTERN INDIA -- TRIBE EUCOSMINI (OLETHREUTINAE) H.S. Rose and H.S. Pooni Department of Zoology, Punjabi University, Patiala, Punjab 147002, India E-mail: [email protected] ABSTRACT Sixteen species belonging to eleven genera viz., Rhopobota Lederer, Acroclita Lederer, Strepsicrates Meyrick, Gibberifera Obraztsov, Loboschiza Diakonoff, Crocidosema Zeller, Epinotia Hübner, Helictophanes Meyrick, Acanthoclita Diakonoff, Ancylis Hübner and Eucosma Hübner belonging to the tribe Eusosmini (Olethreutinae) of the family Tortricidae have been collected from northwestern India and dealt with taxonomically. Key to the presently examined genera has been prepared on the basis of characters such as the labial palpi, antennae, costal fold, anal fold, wing venation and male and female genitalic characteristics. Further, keys to the species of the genera Epinotia Hübner and Eucosma Hübner represented by more than one species have also been furnished. Eucosma pseudostrigulata is being reported as new to science. Species such as Rhopobota grypodes Meyrick, Epinotia corynetes Diakonoff, Acanthoclita iridorphna Meyrick and Eucosma prominens Meyrick are being reported for the first time from India, whereas, Acroclita corinthia Meyrick, Gibberifera glaciata Meyrick, Epinotia canthonias Meyrick, Helictophanes dryocoma Meyrick, Ancylis lutescens Meyrick, Eucosma stereoma Meyrick and E. melanoneura Meyrick have been collected for the first -
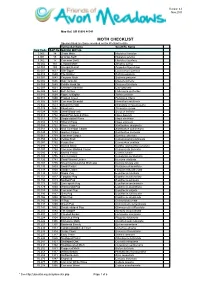
MOTH CHECKLIST Species Listed Are Those Recorded on the Wetland to Date
Version 4.0 Nov 2015 Map Ref: SO 95086 46541 MOTH CHECKLIST Species listed are those recorded on the Wetland to date. Vernacular Name Scientific Name New Code B&F No. MACRO MOTHS 3.005 14 Ghost Moth Hepialus humulae 3.001 15 Orange Swift Hepialus sylvina 3.002 17 Common Swift Hepialus lupulinus 50.002 161 Leopard Moth Zeuzera pyrina 54.008 169 Six-spot Burnet Zygaeba filipendulae 66.007 1637 Oak Eggar Lasiocampa quercus 66.010 1640 The Drinker Euthrix potatoria 68.001 1643 Emperor Moth Saturnia pavonia 65.002 1646 Oak Hook-tip Drepana binaria 65.005 1648 Pebble Hook-tip Drepana falcataria 65.007 1651 Chinese Character Cilix glaucata 65.009 1653 Buff Arches Habrosyne pyritoides 65.010 1654 Figure of Eighty Tethia ocularis 65.015 1660 Frosted Green Polyploca ridens 70.305 1669 Common Emerald Hermithea aestivaria 70.302 1673 Small Emerald Hemistola chrysoprasaria 70.029 1682 Blood-vein Timandra comae 70.024 1690 Small Blood-vein Scopula imitaria 70.013 1702 Small Fan-footed Wave Idaea biselata 70.011 1708 Single-dotted Wave Idaea dimidiata 70.016 1713 Riband Wave Idaea aversata 70.053 1722 Flame Carpet Xanthorhoe designata 70.051 1724 Red Twin-spot Carpet Xanthorhoe spadicearia 70.049 1728 Garden Carpet Xanthorhoe fluctuata 70.061 1738 Common Carpet Epirrhoe alternata 70.059 1742 Yellow Shell Camptogramma bilineata 70.087 1752 Purple Bar Cosmorhoe ocellata 70.093 1758 Barred Straw Eulithis (Gandaritis) pyraliata 70.097 1764 Common Marbled Carpet Chloroclysta truncata 70.085 1765 Barred Yellow Cidaria fulvata 70.100 1776 Green Carpet Colostygia pectinataria 70.126 1781 Small Waved Umber Horisme vitalbata 70.107 1795 November/Autumnal Moth agg Epirrita dilutata agg. -

Redalyc.Delineation of Types of Tortricidae, 1. Types of Oriental
SHILAP Revista de Lepidopterología ISSN: 0300-5267 [email protected] Sociedad Hispano-Luso-Americana de Lepidopterología España Razowski, J.; Wojtusiak, J. Delineation of Types of Tortricidae, 1. Types of Oriental species in the Munich Museum described by A. Diakonoff (Lepidoptera: Tortricidae) SHILAP Revista de Lepidopterología, vol. 37, núm. 146, junio, 2009, pp. 191-208 Sociedad Hispano-Luso-Americana de Lepidopterología Madrid, España Available in: http://www.redalyc.org/articulo.oa?id=45512170009 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative 191-208 Delineation of Types of 31/5/09 15:07 Página 191 SHILAP Revta. lepid., 37 (146), junio 2009: 191-208 CODEN: SRLPEF ISSN:0300-5267 Delineation of Types of Tortricidae, 1. Types of Oriental species in the Munich Museum described by A. Diakonoff (Lepidoptera: Tortricidae) J. Razowski & J. Wojtusiak Abstract The paper consists of the data on 58 primary types and one paratype; colour illustrations of 61 adults are provided. Four new combinations are proposed. KEY WORD: Lepidoptera, Tortricidae, Type species, Diakonoff. Descripción de los tipos de Tortricidae, 1. Tipos de las especies orientales en el Museo de Munich, descritos por A. Diakonoff (Lepidoptera: Tortricidae) Resumen El trabajo consiste en los datos de 58 tipos y un paratipo, se dan ilustraciones a color de 61 adultos. Se proponen cuatro nuevas combinaciones. PALABRAS CLAVE: Lepidoptera, Tortricidae, tipos, Diakonoff. Introduction This is the first paper of our new series on the type specimens of Tortricidae. -
![False Codling Moth, Thaumatotibia (=Cryptophlebia) Leucotreta (Meyrick) [Lepidoptera: Tortricidae]](https://docslib.b-cdn.net/cover/0479/false-codling-moth-thaumatotibia-cryptophlebia-leucotreta-meyrick-lepidoptera-tortricidae-1400479.webp)
False Codling Moth, Thaumatotibia (=Cryptophlebia) Leucotreta (Meyrick) [Lepidoptera: Tortricidae]
Mini Risk Assessment False codling moth, Thaumatotibia (=Cryptophlebia) leucotreta (Meyrick) [Lepidoptera: Tortricidae] Robert C. Venette, Erica E. Davis, Michelle DaCosta, Holly Heisler, & Margaret Larson Department of Entomology, University of Minnesota St. Paul, MN 55108 September 21, 2003 Introduction Thaumatotibia leucotreta is a significant pest of fruit trees and field crops in portions of Africa (CIE 1976, Zhang 1994). Until recently, this pest had been most commonly known as Cryptophlebia leucotreta (Komai 1999). A detailed description of the rationale for the name change is included in this document. For the sake of scientific accuracy, we refer to the false codling moth as T. leucotreta throughout this report. We hope the change does not cause confusion. To our knowledge the risks posed by this pest for US agriculture and native ecosystems have not been evaluated previously in a formal pest risk assessment. Figure 1. Larva and adult of T. leucotreta. Images not to scale. [Larval image from http://www.arc.agric.za/institutes/ itsc/main/avocado/moth.htm; Adult image from Georg Goergen/IITA Insect Museum, Cotonou, Benin as published in (CAB 2000)] 1. Ecological Suitability. Rating: Medium. Thaumatotibia leucotreta is native to the Ethiopian zoogeographic province and presently occurs in much of Sub- Saharan Africa (CIE 1976, CAB 2000). Climates in the area occupied by this pest can be characterized as tropical, dry or temperate (CAB 2000). The currently reported global distribution of T. leucotreta suggests that the pest may be most closely associated with biomes that are generally classified as desert and xeric shrubland, tropical and subtropical grasslands, savannas, and shrubland; and tropical and subtropical moist broadleaf forest. -

Checklist of Texas Lepidoptera Knudson & Bordelon, Jan 2018 Texas Lepidoptera Survey
1 Checklist of Texas Lepidoptera Knudson & Bordelon, Jan 2018 Texas Lepidoptera Survey ERIOCRANIOIDEA TISCHERIOIDEA ERIOCRANIIDAE TISCHERIIDAE Dyseriocrania griseocapitella (Wlsm.) Eriocraniella mediabulla Davis Coptotriche citripennella (Clem.) Eriocraniella platyptera Davis Coptotriche concolor (Zell.) Coptotriche purinosella (Cham.) Coptotriche clemensella (Cham). Coptotriche sulphurea (F&B) NEPTICULOIDEA Coptotriche zelleriella (Clem.) Tischeria quercitella Clem. NEPTICULIDAE Coptotriche malifoliella (Clem.) Coptotriche crataegifoliae (Braun) Ectoedemia platanella (Clem.) Coptotriche roseticola (F&B) Ectoedemia rubifoliella (Clem.) Coptotriche aenea (F&B) Ectoedemia ulmella (Braun) Asterotriche solidaginifoliella (Clem.) Ectoedemia obrutella (Zell.) Asterotriche heliopsisella (Cham.) Ectoedemia grandisella (Cham.) Asterotriche ambrosiaeella (Cham.) Nepticula macrocarpae Free. Asterotriche helianthi (F&B) Stigmella scintillans (Braun) Asterotriche heteroterae (F&B) Stigmella rhoifoliella (Braun) Asterotriche longeciliata (F&B) Stigmella rhamnicola (Braun) Asterotriche omissa (Braun) Stigmella villosella (Clem.) Asterotriche pulvella (Cham.) Stigmella apicialbella (Cham.) Stigmella populetorum (F&B) Stigmella saginella (Clem.) INCURVARIOIDEA Stigmella nigriverticella (Cham.) Stigmella flavipedella (Braun) PRODOXIDAE Stigmella ostryaefoliella (Clem.) Stigmella myricafoliella (Busck) Tegeticula yuccasella (Riley) Stigmella juglandifoliella (Clem.) Tegeticula baccatella Pellmyr Stigmella unifasciella (Cham.) Tegeticula carnerosanella Pellmyr -

Biological Control of Codling Moth (Cydia Pomonella, Lepidoptera: Tortricidae) and Its Role in Integrated Pest Management, with Emphasis on Entomopathogens
VEDALIA 12 (1): 33-60 (2005) MONOGRAPH ISSN 1405-0420 33 BIOLOGICAL CONTROL OF CODLING MOTH (CYDIA POMONELLA, LEPIDOPTERA: TORTRICIDAE) AND ITS ROLE IN INTEGRATED PEST MANAGEMENT, WITH EMPHASIS ON ENTOMOPATHOGENS LAWRENCE A. LACEY & THOMAS R. UNRUH Yakima Agricultural Research Laboratory, USDA-ARS, 5230 Konnowac Pass Road, Wapato, WA 98951, USA. [email protected] _________________________________ ABSTRACT Codling moth, Cydia pomonella, is a worldwide pest of apple and pear. Traditional control methods have been based predominantly on broad spectrum insecticides. Concerns over the safety, environmental impact, and sustainability of synthetic pesticides have stimulated development and use of softer control methods within the integrated pest management (IPM) strategy. Natural enemies (entomopathogens, predators and parasitoids) and their use as biological control agents play key roles in IPM. In this review we summarize the literature on biological control of codling moth and discuss its integration with other control options in orchard IPM. A variety of entomopathogens have been reported from codling moth, but only the codling moth granulovirus (CpGV) and entomopathogenic nematodes (EPNs) have been developed as microbial control agents. CpGV is highly virulent and selective for neonate codling moth larvae, but may require frequent reapplication due to solar inactivation, especially when population densities are high. The EPNs Steinernema feltiae and S. carpocapsae have good potential for control of overwintering cocooned larvae when temperatures are above 10 and 15°C, respectively and adequate moisture is maintained in the orchard for several hours after EPN application. Parasitism by Mastrus ridibundus (Ichneumonidae) in some Washington State orchards can exceed 40% in the year following releases which can further supplement parasitism by Ascogaster quadradentata (Braconidae) that sporadically approaches 25%. -

Grapholita Funebrana
Grapholita funebrana Scientific Name Grapholita funebrana (Treitschke) Synonyms: Carpocapsa funebrana, Cydia funebrana, Enarmonia funebrana, Endopisa funebrana, Grapholita funebrana, Grapholitha funebrana, Laspeyresia cerasana, Laspeyresia funebrana, Opadia funebrana, and Tortrix funebrana. Note: Grapholita funebrana is often incorrectly referred to as Cydia funebrana. The correct generic placement is in Grapholita (see Komai (1999) for more details). Common Names Plum fruit moth, prune moth, red plum maggot Type of Pest Moth Taxonomic Position Class: Insecta, Order: Lepidoptera, Family: Tortricidae Reason for Inclusion in Manual CAPS Target: AHP Prioritized Pest List – 2003 through 2009 Pest Description Grapholita funebrana is able to develop on many wild and cultivated stone fruits and other plants in the family Rosaceae. This pest occurs in Europe, the Middle East, and northern Asia with losses of 25 to 100% reported. The information provided below is from Alford (1978), Bradley et al. (1979), and Whittle (1984). Eggs: Eggs are deposited singly and measure about 0.7 mm (0.28 in.) across by 0.6 mm (0.24 in.) wide, are lenticular to ovate (flattened and slightly elliptical), and are translucent white, becoming yellow as they mature. When they turn yellow, the egg has a central dome-shape area, circled by a flat ring. Eggs are generally laid during June and July at the base of a fruit stalk, hatching in about 10 days. Larvae: At their longest, larvae are about 10 to 12 mm (0.39 to 0.47 in.) long. The head is dark brown to black. The prothorax is pale yellow; while the prothoracic plate is pale brown with the posterior margin mottled darker brown.