Fern Histology: a Focus on Cell Walls and Aspleniaceae
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download Document
African countries and neighbouring islands covered by the Synopsis. S T R E L I T Z I A 23 Synopsis of the Lycopodiophyta and Pteridophyta of Africa, Madagascar and neighbouring islands by J.P. Roux Pretoria 2009 S T R E L I T Z I A This series has replaced Memoirs of the Botanical Survey of South Africa and Annals of the Kirstenbosch Botanic Gardens which SANBI inherited from its predecessor organisations. The plant genus Strelitzia occurs naturally in the eastern parts of southern Africa. It comprises three arborescent species, known as wild bananas, and two acaulescent species, known as crane flowers or bird-of-paradise flowers. The logo of the South African National Biodiversity Institute is based on the striking inflorescence of Strelitzia reginae, a native of the Eastern Cape and KwaZulu-Natal that has become a garden favourite worldwide. It sym- bolises the commitment of the Institute to champion the exploration, conservation, sustain- able use, appreciation and enjoyment of South Africa’s exceptionally rich biodiversity for all people. J.P. Roux South African National Biodiversity Institute, Compton Herbarium, Cape Town SCIENTIFIC EDITOR: Gerrit Germishuizen TECHNICAL EDITOR: Emsie du Plessis DESIGN & LAYOUT: Elizma Fouché COVER DESIGN: Elizma Fouché, incorporating Blechnum palmiforme on Gough Island PHOTOGRAPHS J.P. Roux Citing this publication ROUX, J.P. 2009. Synopsis of the Lycopodiophyta and Pteridophyta of Africa, Madagascar and neighbouring islands. Strelitzia 23. South African National Biodiversity Institute, Pretoria. ISBN: 978-1-919976-48-8 © Published by: South African National Biodiversity Institute. Obtainable from: SANBI Bookshop, Private Bag X101, Pretoria, 0001 South Africa. -

<I>Equisetum Giganteum</I>
Florida International University FIU Digital Commons FIU Electronic Theses and Dissertations University Graduate School 3-24-2009 Ecophysiology and Biomechanics of Equisetum Giganteum in South America Chad Eric Husby Florida International University, [email protected] DOI: 10.25148/etd.FI10022522 Follow this and additional works at: https://digitalcommons.fiu.edu/etd Recommended Citation Husby, Chad Eric, "Ecophysiology and Biomechanics of Equisetum Giganteum in South America" (2009). FIU Electronic Theses and Dissertations. 200. https://digitalcommons.fiu.edu/etd/200 This work is brought to you for free and open access by the University Graduate School at FIU Digital Commons. It has been accepted for inclusion in FIU Electronic Theses and Dissertations by an authorized administrator of FIU Digital Commons. For more information, please contact [email protected]. FLORIDA INTERNATIONAL UNIVERSITY Miami, Florida ECOPHYSIOLOGY AND BIOMECHANICS OF EQUISETUM GIGANTEUM IN SOUTH AMERICA A dissertation submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY in BIOLOGY by Chad Eric Husby 2009 To: Dean Kenneth Furton choose the name of dean of your college/school College of Arts and Sciences choose the name of your college/school This dissertation, written by Chad Eric Husby, and entitled Ecophysiology and Biomechanics of Equisetum Giganteum in South America, having been approved in respect to style and intellectual content, is referred to you for judgment. We have read this dissertation and recommend that it be approved. _______________________________________ Bradley C. Bennett _______________________________________ Jack B. Fisher _______________________________________ David W. Lee _______________________________________ Leonel Da Silveira Lobo O'Reilly Sternberg _______________________________________ Steven F. Oberbauer, Major Professor Date of Defense: March 24, 2009 The dissertation of Chad Eric Husby is approved. -

Epidermal Morphology of the Pinnae of Angiopteris, Danaea, and Maraffia
American Fern Journal 81(2]:44-62 (1991) Epidermal Morphology of the Pinnae of Angiopteris, Danaea, and Maraffia CRISTINAROLLERI,AMVIBLIA DEFERRARI, AND MAR~ADEL CARMENLAVALLE Laboratory of Botany, Museo de La Plata, Paseo del Bosque, 1900 La Plata, Argentina This is a study of adult epidermis morphology in 17 species of Angiopteris Hoffm., Danaea J. E. Smith, and Marattia Swartz. Epidermal patterns, adult stomata, indument, and idioblasts were studied. Hill and Camus (1986) made an overview of characters of some extant species of Marattiales as part of a cladistic study of extant and fossil members of the order. The epidermal characters they used were subsidiary cells of the stomata, dimensions of the stomata, walls of epidermal cells, and idioblasts. The only character of indument they included in their study was the presence or absence of scales. Rolleri et al. (1987) made the first detailed study dealing with pinna and pinnule indument in the Marattiaceae, although Holttum (1978) had made some general comments on petiole and rhizome scales of Angiopteris, illustrating two species. He suggested that Angiopteris pinna trichomes were diagnostic but needed detailed study. Rolleri et al. (1987) strongly pointed out that epidermal characters are diagnostic at the species level in the Marattiaceae and speculated on generic affinities within the Marattiales. Adult epidermis was described according to the terminology of Rolleri and Deferrari (1986) and Rolleri et al. (1987). Adult stomata were described following the criteria of Stace (1965),van Cotthem (1970, 1971),and Wilkinson (1979). Lellinger's (1985) concept of trichomes was adopted, as well as the terminology of Theobald et al. -
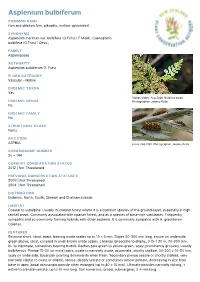
Asplenium Bulbiferum
Asplenium bulbiferum COMMON NAME Hen and chicken fern, pikopiko, mother spleenwort SYNONYMS Asplenium marinum var. bulbifera (G.Forst.) F.Muell.; Caenopteris bulbifera (G.Forst.) Desv.; FAMILY Aspleniaceae AUTHORITY Asplenium bulbiferum G. Forst. FLORA CATEGORY Vascular – Native ENDEMIC TAXON Yes Stokes Valley. Aug 2006. Bulbil on bulbil. ENDEMIC GENUS Photographer: Jeremy Rolfe No ENDEMIC FAMILY No STRUCTURAL CLASS Ferns NVS CODE ASPBUL sorus. Feb 1981. Photographer: Jeremy Rolfe CHROMOSOME NUMBER 2n = 144 CURRENT CONSERVATION STATUS 2012 | Not Threatened PREVIOUS CONSERVATION STATUSES 2009 | Not Threatened 2004 | Not Threatened DISTRIBUTION Endemic. North, South, Stewart and Chatham Islands HABITAT Coastal to subalpine. Usually in lowland forest where it is a common species of the ground-layer, especially in high rainfall areas. Commonly associated with riparian forest, and as a species of base-rich substrates. Frequently sympatric and so commonly forming hybrids with other asplenia. It is commonly sympatric with A. gracillimum Colenso. FEATURES Rhizome short, stout, erect, bearing ovate scales up to 15 × 5 mm. Stipes 50-300 mm long, brown on underside, green above, stout, covered in small brown ovate scales. Laminae lanceolate to elliptic, 0.15-1.20 m, 70-300 mm, bi- to tripinnate, sometimes bearing bulbils. Raches pale green to yellow-green, scaly, prominently grooved, usually bulbiferous. Pinnae 15-30 (or more) pairs, ovate to narrowly ovate, acuminate, shortly stalked, 30-200 × 10-50 mm, scaly on underside, basal pair pointing downwards when fresh. Secondary pinnae sessile or shortly stalked, very narrowly elliptic to ovate or elliptic, obtuse, deeply serrate or sometimes almost pinnate, decreasing in size from base to apex, basal acroscopic pinnule often enlarged (up to 40 × 10 mm). -

Chapter 23: the Early Tracheophytes
Chapter 23 The Early Tracheophytes THE LYCOPHYTES Lycopodium Has a Homosporous Life Cycle Selaginella Has a Heterosporous Life Cycle Heterospory Allows for Greater Parental Investment Isoetes May Be the Only Living Member of the Lepidodendrid Group THE MONILOPHYTES Whisk Ferns Ophioglossalean Ferns Horsetails Marattialean Ferns True Ferns True Fern Sporophytes Typically Have Underground Stems Sexual Reproduction Usually Is Homosporous Fern Have a Variety of Alternative Means of Reproduction Ferns Have Ecological and Economic Importance SUMMARY PLANTS, PEOPLE, AND THE ENVIRONMENT: Sporophyte Prominence and Survival on Land PLANTS, PEOPLE, AND THE ENVIRONMENT: Coal, Smog, and Forest Decline THE OCCUPATION OF THE LAND PLANTS, PEOPLE, AND THE The First Tracheophytes Were ENVIRONMENT: Diversity Among the Ferns Rhyniophytes Tracheophytes Became Increasingly Better PLANTS, PEOPLE, AND THE Adapted to the Terrestrial Environment ENVIRONMENT: Fern Spores Relationships among Early Tracheophytes 1 KEY CONCEPTS 1. Tracheophytes, also called vascular plants, possess lignified water-conducting tissue (xylem). Approximately 14,000 species of tracheophytes reproduce by releasing spores and do not make seeds. These are sometimes called seedless vascular plants. Tracheophytes differ from bryophytes in possessing branched sporophytes that are dominant in the life cycle. These sporophytes are more tolerant of life on dry land than those of bryophytes because water movement is controlled by strongly lignified vascular tissue, stomata, and an extensive cuticle. The gametophytes, however still require a seasonally wet habitat, and water outside the plant is essential for the movement of sperm from antheridia to archegonia. 2. The rhyniophytes were the first tracheophytes. They consisted of dichotomously branching axes, lacking roots and leaves. They are all extinct. -

Molecular Phylogeny of Horsetails (Equisetum) Including Chloroplast Atpb Sequences
J Plant Res DOI 10.1007/s10265-007-0088-x SHORT COMMUNICATION Molecular phylogeny of horsetails (Equisetum) including chloroplast atpB sequences Jean-Michel Guillon Received: 9 November 2006 / Accepted: 21 March 2007 Ó The Botanical Society of Japan and Springer 2007 Abstract Equisetum is a genus of 15 extant species that dependent on vegetative reproduction for persistence and are the sole surviving representatives of the class Sphen- growth. The 15 species of Equisetum are grouped in two opsida. The generally accepted taxonomy of Equisetum subgenera based on morphological characters such as the recognizes two subgenera: Equisetum and Hippochaete. position of stomata: superficial in subgenus Equisetum (E. Two recent phylogenetical studies have independently arvense, E. bogotense, E. diffusum, E. fluviatile, E. pa- questioned the monophyly of subgenus Equisetum. Here, I lustre, E. pratense, E. sylvaticum, and E. telmateia), use original (atpB) and published (rbcL, trnL-trnF, rps4) sunken below the epidermal surface in subgenus Hippo- sequence data to investigate the phylogeny of the genus. chaete (E. giganteum, E. hyemale, E. laevigatum, Analyses of atpB sequences give an unusual topology, with E. myriochaetum, E. ramosissimum, E. scirpoides, and E. bogotense branching within Hippochaete. A Bayesian E. variegatum). A barrier seems to prevent hybridization analysis based on all available sequences yields a tree with between plants of the subgenera Equisetum and Hippo- increased resolution, favoring the sister relationships of chaete (Duckett 1979). E. bogotense with subgenus Hippochaete. Because characters found in the fossil record, such as large stems and persistent sheath teeth, are present in the Keywords Equisetum Á Evolution Á Horsetail Á Phylogeny sole E. -

American Hart's-Tongue Fern
RECOVERY PLAN American ha&s-tongue (Asplenium scolopendrium var. americanum) (Synonym: Phyllitis scoloyendrium var. americana Amencan hart s-tongue fern) U.S. Fish and 1Wildlife Service RECOVERY PLAN for American hart’s-tongue (Asplenium scolopendrium L. var. americanum [Ferna]d]Kartesz and Gandhi [Synonym:Phyllitis scolopendrium (L.) Newman var. americana Fernald]) Prepared by Robert R. Currie Asheville Field Office U.S. Fish and Wildlife Service Asheville, North Carolina for Southeast Region U.S. Fish and Wildlife Service Atlanta, Georgia Jam s W Pulliam,Jr , Director Approved: U.S. K hand Wildlife ServiceV Date: 4’. Recovery plans delineate reasonable actions which are believed to be required to recover and/or protect listed species. Plans are published by the U.S. Fish and Wildlife Service, sometimes prepared with the assistance of recovery teams, contractors, State agencies, and others. Objectives will be attained and any necessary funds made available subject to budgetary and other constraints affecting the parties involved, as well as the need to address other priorities. Recovery plans do not necessarily represent the views nor the official positions or approval of any individuals or agencies involved in the plan formulation, other than the U.S. Fish and Wildlife Service. They represent the official position of the U.S. Fish and Wildlife Service only after they have been signed by the Regional Director or Director as approved. Approved recovery plans are subject to modification as dictated by new findings, changes in species status, and the completion of recovery tasks. Literature citations should read as follows: U.S. Fish and Wildlife Service. 1993. -

A Journal on Taxonomic Botany, Plant Sociology and Ecology Reinwardtia
A JOURNAL ON TAXONOMIC BOTANY, PLANT SOCIOLOGY AND ECOLOGY REINWARDTIA A JOURNAL ON TAXONOMIC BOTANY, PLANT SOCIOLOGY AND ECOLOGY Vol. 13(4): 317 —389, December 20, 2012 Chief Editor Kartini Kramadibrata (Herbarium Bogoriense, Indonesia) Editors Dedy Darnaedi (Herbarium Bogoriense, Indonesia) Tukirin Partomihardjo (Herbarium Bogoriense, Indonesia) Joeni Setijo Rahajoe (Herbarium Bogoriense, Indonesia) Teguh Triono (Herbarium Bogoriense, Indonesia) Marlina Ardiyani (Herbarium Bogoriense, Indonesia) Eizi Suzuki (Kagoshima University, Japan) Jun Wen (Smithsonian Natural History Museum, USA) Managing editor Himmah Rustiami (Herbarium Bogoriense, Indonesia) Secretary Endang Tri Utami Lay out editor Deden Sumirat Hidayat Illustrators Subari Wahyudi Santoso Anne Kusumawaty Reviewers Ed de Vogel (Netherlands), Henk van der Werff (USA), Irawati (Indonesia), Jan F. Veldkamp (Netherlands), Jens G. Rohwer (Denmark), Lauren M. Gardiner (UK), Masahiro Kato (Japan), Marshall D. Sunberg (USA), Martin Callmander (USA), Rugayah (Indonesia), Paul Forster (Australia), Peter Hovenkamp (Netherlands), Ulrich Meve (Germany). Correspondence on editorial matters and subscriptions for Reinwardtia should be addressed to: HERBARIUM BOGORIENSE, BOTANY DIVISION, RESEARCH CENTER FOR BIOLOGY-LIPI, CIBINONG 16911, INDONESIA E-mail: [email protected] REINWARDTIA Vol 13, No 4, pp: 367 - 377 THE NEW PTERIDOPHYTE CLASSIFICATION AND SEQUENCE EM- PLOYED IN THE HERBARIUM BOGORIENSE (BO) FOR MALESIAN FERNS Received July 19, 2012; accepted September 11, 2012 WITA WARDANI, ARIEF HIDAYAT, DEDY DARNAEDI Herbarium Bogoriense, Botany Division, Research Center for Biology-LIPI, Cibinong Science Center, Jl. Raya Jakarta -Bogor Km. 46, Cibinong 16911, Indonesia. E-mail: [email protected] ABSTRACT. WARD AM, W., HIDAYAT, A. & DARNAEDI D. 2012. The new pteridophyte classification and sequence employed in the Herbarium Bogoriense (BO) for Malesian ferns. -

Flora Mediterranea 26
FLORA MEDITERRANEA 26 Published under the auspices of OPTIMA by the Herbarium Mediterraneum Panormitanum Palermo – 2016 FLORA MEDITERRANEA Edited on behalf of the International Foundation pro Herbario Mediterraneo by Francesco M. Raimondo, Werner Greuter & Gianniantonio Domina Editorial board G. Domina (Palermo), F. Garbari (Pisa), W. Greuter (Berlin), S. L. Jury (Reading), G. Kamari (Patras), P. Mazzola (Palermo), S. Pignatti (Roma), F. M. Raimondo (Palermo), C. Salmeri (Palermo), B. Valdés (Sevilla), G. Venturella (Palermo). Advisory Committee P. V. Arrigoni (Firenze) P. Küpfer (Neuchatel) H. M. Burdet (Genève) J. Mathez (Montpellier) A. Carapezza (Palermo) G. Moggi (Firenze) C. D. K. Cook (Zurich) E. Nardi (Firenze) R. Courtecuisse (Lille) P. L. Nimis (Trieste) V. Demoulin (Liège) D. Phitos (Patras) F. Ehrendorfer (Wien) L. Poldini (Trieste) M. Erben (Munchen) R. M. Ros Espín (Murcia) G. Giaccone (Catania) A. Strid (Copenhagen) V. H. Heywood (Reading) B. Zimmer (Berlin) Editorial Office Editorial assistance: A. M. Mannino Editorial secretariat: V. Spadaro & P. Campisi Layout & Tecnical editing: E. Di Gristina & F. La Sorte Design: V. Magro & L. C. Raimondo Redazione di "Flora Mediterranea" Herbarium Mediterraneum Panormitanum, Università di Palermo Via Lincoln, 2 I-90133 Palermo, Italy [email protected] Printed by Luxograph s.r.l., Piazza Bartolomeo da Messina, 2/E - Palermo Registration at Tribunale di Palermo, no. 27 of 12 July 1991 ISSN: 1120-4052 printed, 2240-4538 online DOI: 10.7320/FlMedit26.001 Copyright © by International Foundation pro Herbario Mediterraneo, Palermo Contents V. Hugonnot & L. Chavoutier: A modern record of one of the rarest European mosses, Ptychomitrium incurvum (Ptychomitriaceae), in Eastern Pyrenees, France . 5 P. Chène, M. -

Ana M. Giulietti,1,7,8 Maria José G. Andrade,1,4 Vera L
Rodriguésia 63(1): 001-019. 2012 http://rodriguesia.jbrj.gov.br Molecular phylogeny, morphology and their implications for the taxonomy of Eriocaulaceae Filogenia molecular, morfologia e suas implicações para a taxonomia de Eriocaulaceae Ana M. Giulietti,1,7,8 Maria José G. Andrade,1,4 Vera L. Scatena,2 Marcelo Trovó,6 Alessandra I. Coan,2 Paulo T. Sano,3 Francisco A.R. Santos,1 Ricardo L.B. Borges,1,5 & Cássio van den Berg1 Abstract The pantropical family Eriocaulaceae includes ten genera and c. 1,400 species, with diversity concentrated in the New World. The last complete revision of the family was published more than 100 years ago, and until recently the generic and infrageneric relationships were poorly resolved. However, a multi-disciplinary approach over the last 30 years, using morphological and anatomical characters, has been supplemented with additional data from palynology, chemistry, embryology, population genetics, cytology and, more recently, molecular phylogenetic studies. This led to a reassessment of phylogenetic relationships within the family. In this paper we present new data for the ITS and trnL-F regions, analysed separately and in combination, using maximum parsimony and Bayesian inference. The data confirm previous results, and show that many characters traditionally used for differentiating and circumscribing the genera within the family are homoplasious. A new generic key with characters from various sources and reflecting the current taxonomic changes is presented. Key words: anatomy, ITS, phylogenetics, pollen, trnL-trnF. Resumo Eriocaulaceae é uma família pantropical com dez gêneros e cerca de 1.400 espécies, com centro de diversidade no Novo Mundo, especialmente no Brasil. -

Major Evolutionary Events in the Origin and Diversification of the Fern Genus Polystichum (Dryopteridaceae)1
American Journal of Botany 90(3): 508±514. 2003. MAJOR EVOLUTIONARY EVENTS IN THE ORIGIN AND DIVERSIFICATION OF THE FERN GENUS POLYSTICHUM (DRYOPTERIDACEAE)1 DAMON P. L ITTLE2 AND DAVID S. BARRINGTON3,4 2L. H. Bailey Hortorium, Cornell University, Ithaca, New York 14853-5908 USA; and 3Pringle Herbarium, Department of Botany and Agricultural Biochemistry, The University of Vermont, Burlington, Vermont 05405-0086 USA Recent advances in molecular systematics of the ferns make it possible to address long-standing questions about classi®cation of the major fern genera, such as the worldwide genus Polystichum (Dryopteridaceae), comprising at least 200 species. In this study we examined rbcL sequences and morphological characters from 55 fern taxa: 34 were from Polystichum and 21 were from other genera in the Dryopteridaceae. We found that Phanerophlebia, possibly including Polystichopsis, is the sister group to Polystichum sensu lato (s.l.), including Cyrtomium. Polystichum as commonly recognized is paraphyletic. Our results lead us to suggest recognizing the clade of earliest diverging Polystichum species as a distinct genus (Cyrtomidictyum) and to continue to recognize Cyrtomium as a separate genus, leaving a monophyletic Polystichum sensu stricto (s.s.). We resolved a tropical American clade and an African clade within Polystichum s.s. However, the resemblance between the once-pinnate, bulb-bearing calciphilic species found in Asia and the West Indies appears to be the result of convergent evolution. Optimizing our morphological character transformations onto the combined phylogeny suggests that the common ancestor of Polystichum s.l. and Phanerophlebia had evolved the common features of the alliance, including ciliate petiole-base scales, once-pinnate fronds, ultimate segments with scarious tips, peltate indusia, and microscales. -

List of Vascular Plants of Whenua Hou (Codfish Island)
List of Vascular Plants of Whenua Hou (Codfish Island) Azorella lyallii John Barkla July 2021 This list is based on a visit to Whenua Hou (Codfish Island) by John Barkla 24 July – 7 Aug 2019. Whenua Hou (Codfish Island) lies west of Stewart Island/Rakiura and is c. 1396 hectares in size and rises to a height of 250 m above sea level. Whenua Hou was designated a Nature Reserve in 1986. A central grid reference for the island is NZ Topo50-CH08 900060. The list is supplemented with records of taxa seen by others and recorded in lists by Courtney (1992), Rance (2010), from observations in iNaturalist, from personal communications, and from anonymous and undated records collected from an annotated copy of Hugh Wilson’s field guide ‘Stewart Island Plants’ that resides in the DOC hut on Whenua Hou. Courtney (1992) included records from D.L. Poppelwell 1911, B.A. Fineran 1965, H.D. Wilson 1978 and B. Rance 1990. Rance (2010) included records from P. Johnson 1992, B. Fineran 1965, S. Courtney 1992, R. Cole and J. Hiscock. Where there are multiple records for the same taxa, the most recent observer and date is listed. Plant names follow those used by the New Zealand Plant Conservation Network. Please direct any corrections/additions to John Barkla [email protected]. Observations can also be made directly to iNaturalist Plant lists and references cited Courtney, S. 1992. Checklist of Vascular Plants of Codfish Island. Unpublished list. de Lange, P.J.; Rolfe, J.R.; Barkla, J.W.; Courtney, S.P.; Champion, P.D.; Perrie, L.R.; Beadel, S.M.; Ford, K.A.; Breitwieser,I.; Schonberger, I.; Hindmarsh-Walls, R.; Heenan, P.B.; Ladley, K.