2019 Deschutes River Redband Trout Population Genetics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Environmental Conditions Appendix II
Environmental Conditions Environmental Conditions Appendix II The unique geology, hydrology and climates of the Deschutes River Subbasin create a diverse mix of habitat conditions for fish and wildlife. These populations are linked to the ecosystems in which they live and their health, individual characteristics and abundance reflect the diversity ― and quality ― of their environments. Fish populations, for instance, developed complex life histories through time that responded to the subbasin’s considerable variation in habitat conditions. Stream flows, water temperatures, substrate characteristics and other combined attributes affected anadromous fish distribution within the subbasin and timing of migration. Wildlife population abundance and distribution also developed in response to habitat conditions. Native grasslands once covered vast areas and supported species such as sage grouse, which once occurred exclusively in this habitat. Loss of grassland habitat greatly reduced such populations. Today subbasin habitat conditions continue to influence fish and wildlife production, distribution and survival. These habitats and their attributes ― as well as the aquatic and terrestrial populations they support ― are affected by both natural watershed processes and human activities that influence flow, water quality, upland and riparian conditions and instream habitat. This chapter examines how environmental conditions in the Deschutes watershed affect the subbasin’s fish and wildlife populations. Building on the more general review provided in the Overview, the discussion characterizes the environmental conditions within three watershed areas: the Lower Deschutes watershed (below RM 100), Crooked River watershed, and Upper Deschutes watershed (above RM 100). It characterizes the natural watershed environments, such as geology, climate and hydrology, and the focal fish species they support. -

Oregon Historic Trails Report Book (1998)
i ,' o () (\ ô OnBcox HrsroRrc Tnans Rpponr ô o o o. o o o o (--) -,J arJ-- ö o {" , ã. |¡ t I o t o I I r- L L L L L (- Presented by the Oregon Trails Coordinating Council L , May,I998 U (- Compiled by Karen Bassett, Jim Renner, and Joyce White. Copyright @ 1998 Oregon Trails Coordinating Council Salem, Oregon All rights reserved. No part of this document may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or any information storage or retrieval system, without permission in writing from the publisher. Printed in the United States of America. Oregon Historic Trails Report Table of Contents Executive summary 1 Project history 3 Introduction to Oregon's Historic Trails 7 Oregon's National Historic Trails 11 Lewis and Clark National Historic Trail I3 Oregon National Historic Trail. 27 Applegate National Historic Trail .41 Nez Perce National Historic Trail .63 Oregon's Historic Trails 75 Klamath Trail, 19th Century 17 Jedediah Smith Route, 1828 81 Nathaniel Wyeth Route, t83211834 99 Benjamin Bonneville Route, 1 833/1 834 .. 115 Ewing Young Route, 1834/1837 .. t29 V/hitman Mission Route, 184l-1847 . .. t4t Upper Columbia River Route, 1841-1851 .. 167 John Fremont Route, 1843 .. 183 Meek Cutoff, 1845 .. 199 Cutoff to the Barlow Road, 1848-1884 217 Free Emigrant Road, 1853 225 Santiam Wagon Road, 1865-1939 233 General recommendations . 241 Product development guidelines 243 Acknowledgements 241 Lewis & Clark OREGON National Historic Trail, 1804-1806 I I t . .....¡.. ,r la RivaÌ ï L (t ¡ ...--."f Pðiräldton r,i " 'f Route description I (_-- tt |". -
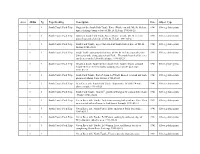
Album Pg Page Heading Description Date Object Type
Area Album Pg Page Heading Description Date Object Type 1 1 South Toutle Pack Trip Origin of the South Fork Toutle River (Wash.) on side Mt. St. Helens; 1941 Silver gelatin prints upper drainage basin at base of Mt. St. Helens; 1941-05-21 1 1 South Toutle Pack Trip Origin of South Fork Toutle River (Wash.) on side Mr. St. Helens; 1941 Silver gelatin prints part of watershed on side of Mt. St. Helens; 1941-05-21 1 1 South Toutle Pack Trip South Fork Toutle; upper watershed of South Fork at base of Mt. St. 1941 Silver gelatin prints Helens; 1941-05-21 1 1 South Toutle Pack Trip South Toutle outwash plain at base of Mt. St. Helens; outwash plain 1941 Silver gelatin prints 200 yards wide along upper south Fork. The north branch of the river can be seen at the left of the picture; 1941-05-21 1 2 South Toutle Pack Trip Bryant at South Toutle shelter; South Fork Toutle; Bryant at South 1941 Silver gelatin prints Toutle Shelter; the first nights stopping place on the pack trip; 1941-05-41 1 2 South Toutle Pack Trip South Fork Toutle; Part of channel of North Branch in broad out wash 1941 Silver gelatin prints plain near Mount Saint Helens; 1941-05-22 1 2 South Toutle Pack Trip Bear Creek trib. South Fork Toutle; Impassable 10' falls 1/4 mile 1941 Silver gelatin prints above mouth; 1941-05-24 1 2 South Toutle Pack Trip South Fork Toutle; Good 2nd growth of Douglas fir around Silver Lake 1941 Silver gelatin prints (Wash.); 1941-06-02 1 3 South Toutle Pack Trip Green River trib. -

Timing of In-Water Work to Protect Fish and Wildlife Resources
OREGON GUIDELINES FOR TIMING OF IN-WATER WORK TO PROTECT FISH AND WILDLIFE RESOURCES June, 2008 Purpose of Guidelines - The Oregon Department of Fish and Wildlife, (ODFW), “The guidelines are to assist under its authority to manage Oregon’s fish and wildlife resources has updated the following guidelines for timing of in-water work. The guidelines are to assist the the public in minimizing public in minimizing potential impacts to important fish, wildlife and habitat potential impacts...”. resources. Developing the Guidelines - The guidelines are based on ODFW district fish “The guidelines are based biologists’ recommendations. Primary considerations were given to important fish species including anadromous and other game fish and threatened, endangered, or on ODFW district fish sensitive species (coded list of species included in the guidelines). Time periods were biologists’ established to avoid the vulnerable life stages of these fish including migration, recommendations”. spawning and rearing. The preferred work period applies to the listed streams, unlisted upstream tributaries, and associated reservoirs and lakes. Using the Guidelines - These guidelines provide the public a way of planning in-water “These guidelines provide work during periods of time that would have the least impact on important fish, wildlife, and habitat resources. ODFW will use the guidelines as a basis for the public a way of planning commenting on planning and regulatory processes. There are some circumstances where in-water work during it may be appropriate to perform in-water work outside of the preferred work period periods of time that would indicated in the guidelines. ODFW, on a project by project basis, may consider variations in climate, location, and category of work that would allow more specific have the least impact on in-water work timing recommendations. -

Volcanic Vistas Discover National Forests in Central Oregon Summer 2009 Celebrating the Re-Opening of Lava Lands Visitor Center Inside
Volcanic Vistas Discover National Forests in Central Oregon Summer 2009 Celebrating the re-opening of Lava Lands Visitor Center Inside.... Be Safe! 2 LAWRENCE A. CHITWOOD Go To Special Places 3 EXHIBIT HALL Lava Lands Visitor Center 4-5 DEDICATED MAY 30, 2009 Experience Today 6 For a Better Tomorrow 7 The Exhibit Hall at Lava Lands Visitor Center is dedicated in memory of Explore Newberry Volcano 8-9 Larry Chitwood with deep gratitude for his significant contributions enlightening many students of the landscape now and in the future. Forest Restoration 10 Discover the Natural World 11-13 Lawrence A. Chitwood Discovery in the Kids Corner 14 (August 4, 1942 - January 4, 2008) Take the Road Less Traveled 15 Larry was a geologist for the Deschutes National Forest from 1972 until his Get High on Nature 16 retirement in June 2007. Larry was deeply involved in the creation of Newberry National Volcanic Monument and with the exhibits dedicated in 2009 at Lava Lands What's Your Interest? Visitor Center. He was well known throughout the The Deschutes and Ochoco National Forests are a recre- geologic and scientific communities for his enthusiastic support for those wishing ation haven. There are 2.5 million acres of forest including to learn more about Central Oregon. seven wilderness areas comprising 200,000 acres, six rivers, Larry was a gifted storyteller and an ever- 157 lakes and reservoirs, approximately 1,600 miles of trails, flowing source of knowledge. Lava Lands Visitor Center and the unique landscape of Newberry National Volcanic Monument. Explore snow- capped mountains or splash through whitewater rapids; there is something for everyone. -

Greater Bend Community Wildfire Protection Plan
Greater Bend Community Wildfire Protection Plan Two Bulls Fire, June 2014 February 2016 Prepared by Project Wildfire 541-322-7129 www.projectwildfire.org [email protected] ª Executive Summary Community Wildfire Protection Plans (CWPPs) are documents that are designed by a local group of stakeholders who are invested in the wildland fire threat to their area. The group of stakeholders typically consists of a representative from the fire department(s), the state, any governing bodies and especially property owners. Each of these representatives should bring their concerns regarding wildland fire to the discussion and propose solutions to their concerns. Although reducing the risk of high intensity wildland fire is the primary motivation behind this plan, managing the larger landscape to restore forest health and more resilient conditions and improving fire response by all fire agencies are also discussed and addressed in the action plan. Continued efforts have been made by county, state and federal land management agencies to reduce the threat of high intensity wildland fires through education and fuels reduction activities on public lands. In addition, private property owners have responded enthusiastically to the defensible space and preparation guidelines and recommendations to reduce hazardous fuels on their own properties by participating in programs such as Firewise and FireFree. All of these activities allow the Greater Bend Area to become a more Fire Adapted Community. Wildland fire is a natural and necessary component of ecosystems across the country. Central Oregon is no exception. Historically, wildland fires have shaped the forests and wildlands valued by residents and visitors. These landscapes however, are now significantly altered due to fire prevention efforts, modern suppression activities and a general lack of large scale fires, resulting in overgrown forests with dense fuels that burn more intensely than in the past. -
22-11 20-10-Se 22-11 20-10-Ne
SUNRIVER N L A T S U N G L U Y N N SUNRA IVER LPOST OFFICE L L I G LY L RI Z Z E D F R C O E IL A N X H T W R E L R D D R N R E G V N RE A A E L T E H T L R ALL E B V N K E LOO IL N E P L IS L D A N T V G E C E N I T L L N B E L A M L N O L A N N C N I B L E G T L A S N A E C N E E BA L L E CK A N M A WO T I D T D ODS N O P O T S P O O A W LN W E Y 153 E R R D L O RANI WAY D IL W M C R ER IG P H WOODLAND P IR T PE C SOUTH CENTURY DR 01 P LN 06 O L L 05 IN I T 04 M 03 W THREE RIVERS 02 AY R 01 06 N 05 E T E L P C ELEMENTARY F L AIRW CENTRAL 03 AY E P G A LN A G ST T E SCHOOL 04 A N LN I MEADOW P S LARK A P E R I K D BOAT LAUNCH E V LN LN N N R A E O L E L E N IV E TERPRIS N N E R N I L O F D P O G R R C ES R T R N D E LN TRAPPER PARKLAND I IV R R LN LN D P PIO R S G NEE IN R LN S N N PR E O S T E P U I S E S H L N R C N D E E R S N E ND PINE B D E L L FI C H L D E T U BOUGH R PA N S L D G PE TE R ABIN LN LN C R INA LN COO R E P SPRING RIVER RD R A R H D P IV O E R R LO Y S CK U E M O M L BOAT LAUNCH R P D IT G AZUSA RD G D N O N I O R IN R A R L R D C Y P B A S R D E N 97 E IN A ¡¢ W R P IV T D D A S Y R E O N N L U L BAKERSFIELD RD L L I E TT LE N RI O VE T P R S D O RD W IVER R O G R L PRIN O S COVINA RD L R G E V I R T 20-10-NW E C 154 L 12 T DOWNEY RD T 07 I R R L 20-O 11-NW 08 A HISTORICAL C V K E 09 20-10-NE N CEMETERY C I 10 0 R 0 11 6 0 ELSINORE RD 07 GU 08 2 12 AD 2 A 09 4 LU WA PE 10 D Y R 0 S 4 D F FONTANA RD R S R D F R D R O L E R R A T D I D E R P T E E D O S M GLENDALE RD S S N O E I P G I L L P N A L I E BOAT LAUNCH R R -

Section 2.5 Water Resources
Exhibit B Section 2.5 Water Resources … Riparian Areas Riparian areas are areas adjacent to rivers, streams, lakes or ponds where there is vegetation that requires free or unbound water or conditions that are more moist than normal. Riparian areas form an interconnected system within a watershed. At the water's edge they define the transition zone between aquatic and terrestrial systems. Riparian areas often contain a diversity of vegetation not found in upland areas. Riparian areas are limited in Deschutes County and are important habitats for both fish and wildlife. The Deschutes County Comprehensive Plan, adopted in 1979 and revised, mapped riparian areas along the following rivers and streams. Table 2.5.5 - Riparian Acreage in Deschutes County Streams Riparian Acres Deschutes River 1,440 Little Deschutes River 2,920 Paulina Creek 846 Indian Ford Creek 573 Tumalo Creek 50 Whychus Creek 47 Fall River 43 Crooked River 38 TOTAL 5,966 Source: Deschutes County/City of Bend River Study 1986 Significant riparian habitat is located in one or more of the following three areas: . The area within 100 feet of the ordinary high water mark of an inventoried river or stream. The 100 foot wide area may contain both riparian vegetation and upland vegetation. Wetlands and flood plain are also frequently within 100 feet of a stream or river. In some cases the riparian vegetation may extend beyond 100 feet from the ordinary high water mark if it is a designated wetland or flood plain. The area adjacent to an inventoried river or stream and located within a flood plain mapped by the Federal Emergency Management Agency and zoned Flood Plain Combining by the County. -

Chapter 5 Supplemental Planning Sections
Chapter 5 Supplemental Sections Planning Section 5.1 Introduction Background This chapter provides material that supplements the other chapters of the Plan. There are no goals or policies in these sections. Purpose The purpose of this chapter is to provide a glossary, list all acknowledged Goal 5 resources in one location (see Section 2.4) and list all Goal Exceptions and Goal 5 inventories. The final section in this Chapter is a table to track all amendments to this Plan. This table will ensure a clear legislative history is maintained. The following information is covered in this chapter. Glossary and Acronyms (Section 5.2) . Goal 5 Water Resources (Section 5.3) . Goal 5 Wildlife Resources (Section 5.4) . Goal 5 Open Space and Scenic Views and Sites Resources (Section 5.5) . Goal 5 Energy Resources (Section 5.6) . Goal 5 Wilderness, Natural Areas and Recreation Trails (Section 5.7) . Goal 5 Surface Mining Resources (Section 5.8) . Goal 5 Cultural and Historic Resources (Section 5.9) . Goal Exception Statements (Section 5.10) . Goal 5 Adopted Ordinances (Section 5.11) . Ordinance History (Section 5.12) 2 DESCHUTES COUNTY COMPREHENSIVE PLAN - 2011 CHAPTER 5 SUPPLEMENTAL SECTIONS SECTION 5.1 INTRODUCTION Section 5.2 Glossary and Acronyms Glossary Note: Terms defined in Deschutes County Code 18.04 (Zoning Code) are not repeated here, but have the same meaning as DCC 18.04. “Agricultural-tourism” or “Agri-tourism” means a commercial enterprise at a working farm or ranch, operated in conjunction with the primary farm or ranch use, conducted for the enjoyment and/or education of visitors, that promotes successful agriculture, generates supplemental income for the owner and complies with Oregon Statute and Rule. -

Jlil~~2T~ 2 DEATH/LELA Tl'bl1cn, BEND O:5LIZL2Z O~/1"/27 Lcel'~TRAL OREGON QAME WARDEN
- - . ...... .. ,. .., ,. '" , BEFORe: 1970 .:JlIl~~2t~ 2 DEATH/LELA tl'Bl1Cn, BEND O:5LIZL2Z _ O~/1"/27 LCEl'~TRAL OREGON QAME WARDEN. CLARENCE ADAMS. V,ILL ABBOTT', RUFUS tl'. 1 AUTO ACCIDENT IN BEND KILL!! HOWARD NOONCHEBTEN I O~/20/;Z7 1 AUTO ACCIDENT INGUEBT ON NCONCHf:STER 06L2l!U:l7 ;Z DEATH/RUFUS A. ABBOTT. REDMOND O~/2~/;Z7 08/11/27 1 REDMOND BOY • .JIM TONEY, KILLED BY F"""l'l-£RS PISTOL ABEL, ANO C. 08/30/27 1 ~ ACCIONET KIL.U!lMRS. W. A. SHELBY. BEND 11/27/~1 ~ DEATHIANO C. ABEl., CROOK COVNTY 09102/27 l'FLOATING ~CDY/MA~ FOUND IN DESCHUTES 09i0"3/27 1 LOGGER. SARAFTNA CAVALON. DIES IN C~MP ACCIDENT ABEL, ESTHER 09/07/27 1 INGUEST ON CAUALON. LOGgE~ ~ILLED !N 4CC!CE~T 12/26/~1 ~ OEATH/MR5. ESTHER ABEL. PRINEVILLE 09/0fl/27 1 TWO BOYS ,~E:PORTf:DLOST IN CASCADES l'</EM fiEr'D 09/0"10?7 1 5EARC~ ~OR LOST BOYS. GUY FERRYfHENRY CRAMER. IN ABREGO, .G:ERALD 5. 09/101'27 1 HOP~ DWINDLES FOR FINDING ~RRY/CRAMER , 1 ~~ STORM ADOS 0 PERILS/BOrS LOST IN C~ecADE~ :O/11/4~ ~ ?FC. ~ERALD 3. ABREGO POSTHUMOVSLY AWARDED B~ONZ 09/12/27 09/12/"27 1 SEARCH ~OR CRAMERIFeR~Y CONTINUES ABRUZZO, FRANK H. 09/13/27 4 ED - MOUNTA!N SEARCH VN~U5T CRITICISM 02/17/69 ;2 DEATH/FRANK ~ ABRUZZO O'U17/27 1 STORMS BLOCK SEARCH FOR FERRY/CRAMER 09/20/27 1 SEARCH FOR LOST YOUTHS RESUMES ACCIDENTS 09/23,27 1 NO TRACe:/L05T CLIMBERS REPORTED ALSO SPECIFIC TYf>ES/ACCIDENTS 09/28/27 1 TRAGIC MISTAKE HELD KEY TO FERRY/CRAMERS DEATH 10/19(06 1 1 BEND FREIGHTER CRUSHED TO DEATH 11/12/27 VETERAN LOGGER. -

Red Butte Cinder Pit Expansion Project Environmental Assessment
Red Butte Cinder Pit Expansion Project United States Environmental Assessment Bend-Fort Rock Ranger District Department of Agriculture Deschutes National Forest Deschutes County, Oregon Forest Service February 2015 Township 18 South, Range 11 East, Section 28 Willamette Meridian For More Information Contact: Beth Peer, Environmental Coordinator 63095 Deschutes Market Road Bend, OR 97701 Phone: 541-383-4769 [email protected] Red Butte Pit Expansion EA The U.S. Department of Agriculture (USDA) prohibits discrimination in all its programs and activities on the basis of race, color, national origin, gender, religion, age, disability, political beliefs, sexual orientation, or marital or family status. (Not all prohibited bases apply to all programs.) Persons with disabilities who require alternative means for communication of program information (Braille, large print, audiotape, etc.) should contact USDA’s TARGET Center at (202) 720-2600 (voice and TDD). To file a complaint of discrimination, write USDA, Director, Office of Civil Rights, Room 326-W, Whitten Building, 14th and Independence Avenue, SW, Washington, DC 20250-9410 or call (202) 720-5964 (voice and TDD). USDA is an equal opportunity provider and employer. Red Butte Pit Expansion EA TABLE OF CONTENTS List of Figures ............................................................................................................................................... 1 List of Tables ............................................................................................................................................... -

RECONNAISSANCE INVESTIGATION of SEDIMENT DISTRIBUTION, EROSION, and TRANSPORT in the UPPER DESCHUTES RIVER, DESCHUTES COUNTY, OREGON, NOVEMBER 1986 by Kenneth A
RECONNAISSANCE INVESTIGATION OF SEDIMENT DISTRIBUTION, EROSION, AND TRANSPORT IN THE UPPER DESCHUTES RIVER, DESCHUTES COUNTY, OREGON, NOVEMBER 1986 By Kenneth A. Cameron and Jon J. Major U. S. GEOLOGICAL SURVEY Water-Resources Investigations Report 87-4114 Prepared in cooperation with the U. S. FOREST SERVICE Vancouver, Washington 1987 DEPARTMENT OF THE INTERIOR DONALD PAUL HODEL, Secretary U.S. GEOLOGICAL SURVEY Dallas L. Peck, Director For additional information Copies of this report may be write to: purchased from: U.S. Geological Survey U. S. Geological Survey 5400 MacArthur Boulevard Books and Open-file Reports Vancouver, Washington 98661 Federal Center, Building 810 Box 25425 Denver, Colorado 80225 11 CONTENTS Page Abstract ---------------------------------------------------------- 1 Introduction ------------------------------------------------------ 2 Statement of problem ----------------------------------------- 2 Purpose and scope -------------------------------------------- 3 Method of investigation -------------------------------------- 3 General stratigraphy ---------------------------------------------- 6 River history ----------------------------------------------------- 7 Granulometric data ------------------------------------------------ 9 Erosion ----------------------------------------------------------- 13 Dendrochronologic determination of bank-erosion rates -------- 13 Riparian area ------------------------------------------------ 14 Channel area ------------------------------------------------- 14 Bedload transport