Download This File
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ARTICLE X. LANDSCAPING Sec 8-447. Purpose. the City of Del Rio
ARTICLE X. LANDSCAPING Sec 8-447. Purpose. The City of Del Rio experiences frequent droughts and is in a semi-arid climatic zone; therefore, it is the purpose of this article to: (1) Encourage the use of drought resistant plants and landscaping techniques that do not consume large quantities of water. Plants native to Southern Texas/Coahuila Desert are recommended. (2) Establish requirements for the installation and maintenance of landscaping on developed commercial properties in order to improve, protect, and preserve the appearance, character and value of such properties and their surrounding neighborhoods and thereby promote the public health, safety and general welfare of the citizens of Del Rio. More specifically, it is the purpose of this article to: (a) Aid in stabilizing the environment's ecological balance by contributing to the process of air purification, oxygen regeneration, storm water runoff retardation and groundwater recharge; (b) Reduce soil erosion by slowing storm water runoff; (c) Aid in the abatement of noise, glare and heat; (d) Aid in energy conservation; (e) Provide visual buffering and provide contrast and relief from the built-up environment; and (f) Protect and enhance property value and public and private investment and enhance the beautification of the city. (3) Contribute to and enhance the economic welfare of the city and the quality of life of citizens and visitors through the following: a. Promote the image of the southwestern border environment; and b. Create an attractive appearance along city streets -
Additional Information
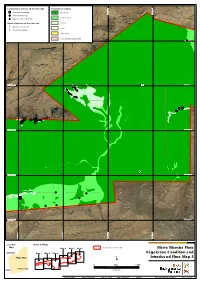
Current Survey Introduced Flora Records Vegetation Condition *Acetosa vesicaria Excellent 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 -

Seed Ecology Iii
SEED ECOLOGY III The Third International Society for Seed Science Meeting on Seeds and the Environment “Seeds and Change” Conference Proceedings June 20 to June 24, 2010 Salt Lake City, Utah, USA Editors: R. Pendleton, S. Meyer, B. Schultz Proceedings of the Seed Ecology III Conference Preface Extended abstracts included in this proceedings will be made available online. Enquiries and requests for hardcopies of this volume should be sent to: Dr. Rosemary Pendleton USFS Rocky Mountain Research Station Albuquerque Forestry Sciences Laboratory 333 Broadway SE Suite 115 Albuquerque, New Mexico, USA 87102-3497 The extended abstracts in this proceedings were edited for clarity. Seed Ecology III logo designed by Bitsy Schultz. i June 2010, Salt Lake City, Utah Proceedings of the Seed Ecology III Conference Table of Contents Germination Ecology of Dry Sandy Grassland Species along a pH-Gradient Simulated by Different Aluminium Concentrations.....................................................................................................................1 M Abedi, M Bartelheimer, Ralph Krall and Peter Poschlod Induction and Release of Secondary Dormancy under Field Conditions in Bromus tectorum.......................2 PS Allen, SE Meyer, and K Foote Seedling Production for Purposes of Biodiversity Restoration in the Brazilian Cerrado Region Can Be Greatly Enhanced by Seed Pretreatments Derived from Seed Technology......................................................4 S Anese, GCM Soares, ACB Matos, DAB Pinto, EAA da Silva, and HWM Hilhorst -

AMERICAN 0/ AŒDICUNAL 'Ö^ PLANTS of COMMERCIAL Importajsfce
AMERICAN 0/ AŒDICUNAL 'Ö^ PLANTS OF COMMERCIAL IMPORTAJSfCE i>i :<ic MISCELLANEOUS .,,„ PUBLICATION No.77 ^'' UNITED STATES DEPARTMENT OF AMONG THE WILD PLANTS of the United States are many £\ that have long been used m the practice of medicine, some only locally and to a minor extent, but others in sufficient quantity to make them commercially important. The collection of such plants for the crude-drug market provides a livelihood for many people in rural communities, especially in those regions where the native flora has not been disturbed by agricultural or industrial expansion and urban development. There is an active interest in the collection of medicinal i)lants because it appeals to many people as an easy means of making money. However, it frequently requires hard work, and the returns, on the whole, are very moderate. Of the many plants reported to possess medicinal properties, relatively few are marketable, and some of these are required only in small quantities. Persons without previous experience in collecting medicinal plants should first ascertain which of the marketable plants are to be found in their own locality and then learn to recognize them. Before undertaking the collection of large quantities, samples of the bark, root, herb, or other available material should be submitted to reliable dealers in crude drugs to ascertain the market requirements at the time and the prevailing prices. To persons without botanical training it is difficult to describe plants in sufficient detail to make identification possible unless such descriptions are accompanied by illustrations. It is the purpose of this publication to assist those interested in collecting medicinal plants to identify such plants and to furnish other useful information in connection with the work. -

The Ingham County News
// you seek a delightful Those li'hosc care c.vlends peninsula, look about you. not jar ahead will find lheir —Motto of MicliigaiL INGHAM [TY NEWS I troubles near al hand. Seventy-fourtlt ycai-, No. 35 INGHAM COUNTY NEWS, MASON, MICHIGAN, THURSDAY, AUGUST 31, 1933 Twelve Pages ON WELFARE BOARD Ancient Tile Found Cucumber Growers Get MiyORGANIZAIilO By Drain Contractor GOVERIENI ADDS CAS Benefit Of New Code MY FAIR PAID lY; PIONEER FARMER USED ILXND- WILSON PACKING Co. nooSTS HMMGEOFllFM ONPE»S;l\PmNIS tAlADE TILE ON LAND. 10 HOG PRICES 10 SPUR PKIOE ro GItOWERS. FAIR OFFICIALS PLEASED Fred Gailey of IDansviJle, a drnin Cucuinber growers selling their crop WORK IN SIX lOlSHIPS ASKIMMEDIAIEPAYiN contractor, uncovered an antique this to the Wilson Packing company in week on the If'ay Townsend farm. He EIING OF SURPLUS Mason are not only getting an excep BY AIIENDANCE RECORD dug up a hand-ninde tile that has seen tional yield this yoar but they are re IIE/VDQUARTBHS IN LANSING, HEAD TAX UEGISTKATIoN DE service of at least GO years. Old- BONUS OF p.m PAID FOR LIGHT ceiving the benefit of a 25 per cent wm COUNTY FAIR WILL SHOW BItANOlI OtiTlOICS PLANNEIJ. LAYED BY STATE. tiniers say that the type of tile un EST PIGS. iscrensD over tho contract price. Tho A SHI ALL PROFIT. covered by Mr. Gailey was rai'c even price increase was a voluntary contri Sixiliil Scrvico Worker To Ailmliilwti'r C. Hdss Ilillliiri^ Cfxiiity Cleric, Itc- 50 yeai's ago. Tho tile is not cylindri bution of the pickle nianufaoturevs cal. -

Outline of Angiosperm Phylogeny
Outline of angiosperm phylogeny: orders, families, and representative genera with emphasis on Oregon native plants Priscilla Spears December 2013 The following listing gives an introduction to the phylogenetic classification of the flowering plants that has emerged in recent decades, and which is based on nucleic acid sequences as well as morphological and developmental data. This listing emphasizes temperate families of the Northern Hemisphere and is meant as an overview with examples of Oregon native plants. It includes many exotic genera that are grown in Oregon as ornamentals plus other plants of interest worldwide. The genera that are Oregon natives are printed in a blue font. Genera that are exotics are shown in black, however genera in blue may also contain non-native species. Names separated by a slash are alternatives or else the nomenclature is in flux. When several genera have the same common name, the names are separated by commas. The order of the family names is from the linear listing of families in the APG III report. For further information, see the references on the last page. Basal Angiosperms (ANITA grade) Amborellales Amborellaceae, sole family, the earliest branch of flowering plants, a shrub native to New Caledonia – Amborella Nymphaeales Hydatellaceae – aquatics from Australasia, previously classified as a grass Cabombaceae (water shield – Brasenia, fanwort – Cabomba) Nymphaeaceae (water lilies – Nymphaea; pond lilies – Nuphar) Austrobaileyales Schisandraceae (wild sarsaparilla, star vine – Schisandra; Japanese -

Psorothamnus Arborescens Var. Pubescens (Parish) Barneby Marble Canyon Dalea
TOC Page | 117 Psorothamnus arborescens var. pubescens (Parish) Barneby Marble Canyon Dalea Family: Fabaceae Synonyms: None NESL Status: G4 Federal Status: None Plant Description: Armed shrubs 4-10 dm tall; leaves 1.4-3.8cm long, leaflets 7-15, glandular beneath, strigose on both sides. Racemes 11-21 flowered, 1.8-4.5cm long. Calyx 8-10mm long, the tube 3.8- 4.8mm long, 10-ribbed, villous, the teeth 3.6-5.2mm long, linear-lanceolate, as long as the tube; flowers 8.1-10.6 mm long, indigo; valves of pods with large, round, discrete yellowish or orange blister glands, pubescent between blister glands. Flowering and fruiting from May to June. Similar species: Differs consistently from P. fremontii only in the ornamentation of the pod. P. fremontii has small orange glands that are confluent lengthwise into crowded ridges towards the pod’s beak. P. arborescens has large round blister glands separated from one another by spaces as wide as their diameter. Habitat: On soils derived from the Moenkopi Formation in mixed desert shrub communities between 3400 – 4900ft Distribution: Endemic Northern Coconino County, AZ, in the vicinity of Marble Canyon. Navajo Nation Distribution: Currently only known from Navajo Springs area, south of Navajo Bridge. Potential Navajo Nation Distribution: Lee’s Backbone to Bitter Springs. Recommended Survey Period: Proper identification is only possible during the flowering and fruiting period in May and June. Recommended Avoidance: A 200 ft buffer zone is recommended to avoid disturbance; may be more or less depending on size and nature of the project. References: Arizona Rare Plant Committee. -

Pines in the Arboretum
UNIVERSITY OF MINNESOTA MtJ ARBORETUM REVIEW No. 32-198 PETER C. MOE Pines in the Arboretum Pines are probably the best known of the conifers native to The genus Pinus is divided into hard and soft pines based on the northern hemisphere. They occur naturally from the up the hardness of wood, fundamental leaf anatomy, and other lands in the tropics to the limits of tree growth near the Arctic characteristics. The soft or white pines usually have needles in Circle and are widely grown throughout the world for timber clusters of five with one vascular bundle visible in cross sec and as ornamentals. In Minnesota we are limited by our cli tions. Most hard pines have needles in clusters of two or three mate to the more cold hardy species. This review will be with two vascular bundles visible in cross sections. For the limited to these hardy species, their cultivars, and a few hy discussion here, however, this natural division will be ignored brids that are being evaluated at the Arboretum. and an alphabetical listing of species will be used. Where neces Pines are readily distinguished from other common conifers sary for clarity, reference will be made to the proper groups by their needle-like leaves borne in clusters of two to five, of particular species. spirally arranged on the stem. Spruce (Picea) and fir (Abies), Of the more than 90 species of pine, the following 31 are or for example, bear single leaves spirally arranged. Larch (Larix) have been grown at the Arboretum. It should be noted that and true cedar (Cedrus) bear their leaves in a dense cluster of many of the following comments and recommendations are indefinite number, whereas juniper (Juniperus) and arborvitae based primarily on observations made at the University of (Thuja) and their related genera usually bear scalelikie or nee Minnesota Landscape Arboretum, and plant performance dlelike leaves that are opposite or borne in groups of three. -

December 2012 Number 1
Calochortiana December 2012 Number 1 December 2012 Number 1 CONTENTS Proceedings of the Fifth South- western Rare and Endangered Plant Conference Calochortiana, a new publication of the Utah Native Plant Society . 3 The Fifth Southwestern Rare and En- dangered Plant Conference, Salt Lake City, Utah, March 2009 . 3 Abstracts of presentations and posters not submitted for the proceedings . 4 Southwestern cienegas: Rare habitats for endangered wetland plants. Robert Sivinski . 17 A new look at ranking plant rarity for conservation purposes, with an em- phasis on the flora of the American Southwest. John R. Spence . 25 The contribution of Cedar Breaks Na- tional Monument to the conservation of vascular plant diversity in Utah. Walter Fertig and Douglas N. Rey- nolds . 35 Studying the seed bank dynamics of rare plants. Susan Meyer . 46 East meets west: Rare desert Alliums in Arizona. John L. Anderson . 56 Calochortus nuttallii (Sego lily), Spatial patterns of endemic plant spe- state flower of Utah. By Kaye cies of the Colorado Plateau. Crystal Thorne. Krause . 63 Continued on page 2 Copyright 2012 Utah Native Plant Society. All Rights Reserved. Utah Native Plant Society Utah Native Plant Society, PO Box 520041, Salt Lake Copyright 2012 Utah Native Plant Society. All Rights City, Utah, 84152-0041. www.unps.org Reserved. Calochortiana is a publication of the Utah Native Plant Society, a 501(c)(3) not-for-profit organi- Editor: Walter Fertig ([email protected]), zation dedicated to conserving and promoting steward- Editorial Committee: Walter Fertig, Mindy Wheeler, ship of our native plants. Leila Shultz, and Susan Meyer CONTENTS, continued Biogeography of rare plants of the Ash Meadows National Wildlife Refuge, Nevada. -

Pinus Parviflora Japanese White Pine1 Edward F
Fact Sheet ST-470 October 1994 Pinus parviflora Japanese White Pine1 Edward F. Gilman and Dennis G. Watson2 INTRODUCTION Japanese White Pine creates a striking landscape element wherever it is used (Fig. 1). Often seen as a dense, conical form when young, Japanese White Pine develops into a 25 to 50-foot-tall, graceful, irregularly- shaped tree, with an equal or greater spread, and a broad, flattened canopy. The 1 to 2.5-inch-long needles are stiff and twisted, forming blue/green tufts of foliage at branch tips, and creating an overall fine texture to the tree’s silhouette. The brownish-red cones are one to four inches long and persist on the tree for six to seven years. GENERAL INFORMATION Scientific name: Pinus parviflora Figure 1. Young Japanese White Pine. Pronunciation: PIE-nus par-vih-FLOR-uh Common name(s): Japanese White Pine Texture: fine Family: Pinaceae USDA hardiness zones: 4B through 7A (Fig. 2) Foliage Origin: not native to North America Uses: Bonsai; screen; specimen; no proven urban Leaf arrangement: alternate; spiral (Fig. 3) tolerance Leaf type: simple Availability: somewhat available, may have to go out Leaf margin: entire of the region to find the tree Leaf shape: needle-like (filiform) Leaf venation: parallel DESCRIPTION Leaf type and persistence: evergreen; fragrant; needle leaf evergreen Height: 25 to 50 feet Leaf blade length: 2 to 4 inches; less than 2 inches Spread: 25 to 50 feet Leaf color: blue or blue-green; green Crown uniformity: irregular outline or silhouette Fall color: no fall color change Crown shape: spreading; pyramidal Fall characteristic: not showy Crown density: dense Growth rate: slow 1. -

The Sam Eskin Collection, 1939-1969, AFC 1999/004
The Sam Eskin Collection, 1939 – 1969 AFC 1999/004 Prepared by Sondra Smolek, Patricia K. Baughman, T. Chris Aplin, Judy Ng, and Mari Isaacs August 2004 Library of Congress American Folklife Center Washington, D. C. Table of Contents Collection Summary Collection Concordance by Format Administrative Information Provenance Processing History Location of Materials Access Restrictions Related Collections Preferred Citation The Collector Key Subjects Subjects Corporate Subjects Music Genres Media Formats Recording Locations Field Recording Performers Correspondents Collectors Scope and Content Note Collection Inventory and Description SERIES I: MANUSCRIPT MATERIAL SERIES II: SOUND RECORDINGS SERIES III: GRAPHIC IMAGES SERIES IV: ELECTRONIC MEDIA Appendices Appendix A: Complete listing of recording locations Appendix B: Complete listing of performers Appendix C: Concordance listing original field recordings, corresponding AFS reference copies, and identification numbers Appendix D: Complete listing of commercial recordings transferred to the Motion Picture, Broadcast, and Recorded Sound Division, Library of Congress 1 Collection Summary Call Number: AFC 1999/004 Creator: Eskin, Sam, 1898-1974 Title: The Sam Eskin Collection, 1938-1969 Contents: 469 containers; 56.5 linear feet; 16,568 items (15,795 manuscripts, 715 sound recordings, and 57 graphic materials) Repository: Archive of Folk Culture, American Folklife Center, Library of Congress, Washington, D.C. Summary: This collection consists of materials gathered and arranged by Sam Eskin, an ethnomusicologist who recorded and transcribed folk music he encountered on his travels across the United States and abroad. From 1938 to 1952, the majority of Eskin’s manuscripts and field recordings document his growing interest in the American folk music revival. From 1953 to 1969, the scope of his audio collection expands to include musical and cultural traditions from Latin America, the British Isles, the Middle East, the Caribbean, and East Asia. -

Tree and Tree-Like Species of Mexico: Asteraceae, Leguminosae, and Rubiaceae
Revista Mexicana de Biodiversidad 84: 439-470, 2013 Revista Mexicana de Biodiversidad 84: 439-470, 2013 DOI: 10.7550/rmb.32013 DOI: 10.7550/rmb.32013439 Tree and tree-like species of Mexico: Asteraceae, Leguminosae, and Rubiaceae Especies arbóreas y arborescentes de México: Asteraceae, Leguminosae y Rubiaceae Martin Ricker , Héctor M. Hernández, Mario Sousa and Helga Ochoterena Herbario Nacional de México, Departamento de Botánica, Instituto de Biología, Universidad Nacional Autónoma de México. Apartado postal 70- 233, 04510 México D. F., Mexico. [email protected] Abstract. Trees or tree-like plants are defined here broadly as perennial, self-supporting plants with a total height of at least 5 m (without ascending leaves or inflorescences), and with one or several erect stems with a diameter of at least 10 cm. We continue our compilation of an updated list of all native Mexican tree species with the dicotyledonous families Asteraceae (36 species, 39% endemic), Leguminosae with its 3 subfamilies (449 species, 41% endemic), and Rubiaceae (134 species, 24% endemic). The tallest tree species reach 20 m in the Asteraceae, 70 m in the Leguminosae, and also 70 m in the Rubiaceae. The species-richest genus is Lonchocarpus with 67 tree species in Mexico. Three legume genera are endemic to Mexico (Conzattia, Hesperothamnus, and Heteroflorum). The appendix lists all species, including their original publication, references of taxonomic revisions, existence of subspecies or varieties, maximum height in Mexico, and endemism status. Key words: biodiversity, flora, tree definition. Resumen. Las plantas arbóreas o arborescentes se definen aquí en un sentido amplio como plantas perennes que se pueden sostener por sí solas, con una altura total de al menos 5 m (sin considerar hojas o inflorescencias ascendentes) y con uno o varios tallos erectos de un diámetro de al menos 10 cm.