Fetal Awareness Review of Research and Recommendations for Practice
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Risk Factors Associated with Maternal Age and Other Parameters in Assisted Reproductive Technologies - a Brief Review
Available online at www.pelagiaresearchlibrary.com Pelagia Research Library Advances in Applied Science Research, 2017, 8(2):15-19 ISSN : 0976-8610 CODEN (USA): AASRFC Risk Factors Associated with Maternal Age and Other Parameters in Assisted Reproductive Technologies - A Brief Review Shanza Ghafoor* and Nadia Zeeshan Department of Biochemistry and Biotechnology, University of Gujrat, Hafiz Hayat Campus, Gujrat, Punjab, Pakistan ABSTRACT Assisted reproductive technology is advancing at fast pace. Increased use of ART (Assisted reproductive technology) is due to changing living standards which involve increased educational and career demand, higher rate of infertility due to poor lifestyle and child conceivement after second marriage. This study gives an overview that how advancing age affects maternal and neonatal outcomes in ART (Assisted reproductive technology). Also it illustrates how other factor like obesity and twin pregnancies complicates the scenario. The studies find an increased rate of preterm birth .gestational hypertension, cesarean delivery chances, high density plasma, Preeclampsia and fetal death at advanced age. The study also shows the combinatorial effects of mother age with number of embryos along with number of good quality embryos which are transferred in ART (Assisted reproductive technology). In advanced age women high clinical and multiple pregnancy rate is achieved by increasing the number along with quality of embryos. Keywords: Reproductive techniques, Fertility, Lifestyle, Preterm delivery, Obesity, Infertility INTRODUCTION Assisted reproductive technology actually involves group of treatments which are used to achieve pregnancy when patients are suffering from issues like infertility or subfertility. The treatments can involve invitro fertilization [IVF], intracytoplasmic sperm injection [ICSI], embryo transfer, egg donation, sperm donation, cryopreservation, etc., [1]. -

The Effects of Alcohol in Newborns Efeitos Do Álcool No Recém-Nascido
REVIEW The effects of alcohol in newborns Efeitos do álcool no recém-nascido Maria dos Anjos Mesquita* ABSTRACT alcoólicas leva a prejuízos individuais, para a sua família e para The purpose of this article was to present a review of the effects toda a sociedade. Apesar disso, a dificuldade do seu diagnóstico e of alcohol consumption by pregnant mothers on their newborn. a inexperiência dos profissionais de saúde faz com que o espectro Definitions, prevalence, pathophysiology, clinical features, diagnostic dessas lesões seja pouco lembrado e até desconhecido. As lesões criteria, follow-up, treatment and prevention were discussed. A causadas pela ação do álcool no concepto são totalmente prevenidas search was performed in Medline, LILACS, and SciELO databases se a gestante não consumir bebidas alcoólicas durante a gestação. using the following terms: “fetus”, “newborn”, “pregnant woman”, Assim, é fundamental a detecção das mulheres consumidoras “alcohol”, “alcoholism”, “fetal alcohol syndrome”, and “alcohol- de álcool durante a gravidez e o desenvolvimento de programas related disorders”. Portuguese and English articles published from específicos de alerta sobre as consequências do álcool durante a 2000 to 2009 were reviewed. The effects of alcohol consumed by gestação e amamentação. pregnant women on newborns are extremely serious and occur frequently; it is a major issue in Public Health worldwide. Fetal alcohol Descritores: Bebidas alcoólicas/efeitos adversos; Feto; Recém-nascido; spectrum disorders cause harm to individuals, their families, and the Síndrome alcoólica fetal; Transtornos relacionados ao uso de álcool entire society. Nevertheless, diagnostic difficulties and inexperience of healthcare professionals result in such damage, being remembered rarely or even remaining uncovered. -

Extended Abstract
Extended abstract Effect of intrauterine development and nutritional status on perinatal, intrauterine and neonatal mortality 1 Péter Berkő, 2 Kálmán Joubert, 3 Éva Gárdos, 4 Gyula Gyenis 1Faculty of Healthcare, Miskolc University, BAZ County and University Teaching Hospital Miskolc, Hungary, - 2Demographic Research Institute, Hungarian Central Statistical Office, Budapest, - 3Hungarian Central Statistical Office, Budapest, - 4Department of Biological Anthropology, Faculty of Science, Eötvös Lorand University, Budapest, Hungary At least 50-60% of 3 million of intrauterine and near 4 million deaths that occur worldwide every year are associated with low birth weight, caused by intrauterine growth restriction, preterm delivery, and genetic abnormalities. Fetal growth restriction is the second leading cause of perinatal morbidity and mortality. The authors study to what extent bodily development and nutritional status influence the viability, or perinatal mortality of foetuses and neonates. In the present study the authors describe their novel method, the MDN system (MDN: Maturity, Development, Nutritional status) and its application: 1./ to determine the nutritional status of a neonate on the basis of its gestational age, length and weight development considered simultaneously; - 2./ to differentiate the most viable and the most endangered neonates on the basis of their development and nutritional status; - 3./ to demonstrate the influence of a neonate’s nutritional status by the gestational age on its perinatal mortality. Method – the MDN system The authors have developed a new method, the MDN system (MDN: Maturity, Development, Nutritional status) to determine the weight and length standard positions of neonates in relation to reference standards on the basis of their gestational ages, birth weights and lengths. -

Bivariate Analysis of Birth Weight and Gestational Age Depending on Environmental Exposures: Bayesian Distributional Regression with Copulas
Bivariate Analysis of Birth Weight and Gestational Age Depending on Environmental Exposures: Bayesian Distributional Regression with Copulas Jonathan Rathjens1, Arthur Kolbe2, Jürgen Hölzer2, Katja Ickstadt1, and Nadja Klein3 1Technische Universität Dortmund, 2Ruhr-Universität Bochum, 3Humboldt-Universität zu Berlin April 30, 2021 Abstract In this article, we analyze perinatal data with birth weight (BW) as primarily interesting response variable. Gestational age (GA) is usually an important covariate and included in polynomial form. However, in opposition to this univariate regression, bivariate modeling of BW and GA is recommended to distinguish effects on each, on both, and between them. Rather than a parametric bivariate distribution, we apply conditional copula regression, where marginal distributions of BW and GA (not necessarily of the same form) can be estimated independently, and where the dependence structure is modeled conditional on the covariates separately from these marginals. In the resulting distributional regression models, all parame- ters of the two marginals and the copula parameter are observation-specific. Besides biometric and obstetric information, data on drinking water contamination and maternal smoking are in- cluded as environmental covariates. While the Gaussian distribution is suitable for BW, the skewed GA data are better modeled by the three-parametric Dagum distribution. The Clay- ton copula performs better than the Gumbel and the symmetric Gaussian copula, indicating lower tail dependence (stronger dependence when both variables are low), although this non- linear dependence between BW and GA is surprisingly weak and only influenced by Cesarean arXiv:2104.14243v1 [stat.ME] 29 Apr 2021 section. A non-linear trend of BW on GA is detected by a classical univariate model that is polynomial with respect to the effect of GA. -

FASD Effects of Alcohol on a Fetus
EFFECTS OF ALCOHOL ON A FETUS “Of all the substances of abuse (including cocaine, heroin, and marijuana), alcohol produces by far the most serious neurobehavioral effects in the fetus.” —Institute of Medicine Report to Congress, 19961 Prenatal exposure to alcohol can damage a fetus at any time, causing problems that persist throughout the individual’s life. There is no known safe level of alcohol use in pregnancy. WHAT IS THE SCOPE OF THE PROBLEM? identified in virtually every part of the body, including the brain, face, eyes, ears, heart, kidneys, and bones. No Alcohol is one of the most dangerous teratogens, which single mechanism can account for all the problems that are substances that can damage a developing fetus.1 Every alcohol causes. Rather, alcohol sets in motion many time a pregnant woman has a drink, her unborn child processes at different sites in the developing fetus: has one, too. Alcohol, like carbon monoxide from cigarettes, passes easily through the placenta from the • Alcohol can trigger cell death in a number of ways, mother's bloodstream into her baby's blood (See Figure causing different parts of the fetus to develop abnormally. 1)—and puts her fetus at risk of having a fetal alcohol • Alcohol can disrupt the way nerve cells develop, travel spectrum disorder (FASD). The blood alcohol level to form different parts of the brain, and function. (BAC) of the fetus becomes equal to or greater than the blood alcohol level of the mother. Because the fetus • By constricting the blood vessels, alcohol interferes with cannot break down alcohol the way an adult can, its BAC blood flow in the placenta, which hinders the delivery 2 remains high for a longer period of time. -

GMEC) Strategic Clinical Networks Reduced Fetal Movement (RFM
Greater Manchester & Eastern Cheshire (GMEC) Strategic Clinical Networks Reduced Fetal Movement (RFM) in Pregnancy Guidelines March 2019 Version 1.3a GMEC RFM Guideline FINAL V1.3a 130619 Issue Date 15/02/2019 Version V1.3a Status Final Review Date Page 1 of 19 Document Control Ownership Role Department Contact Project Clinical Lead Manchester Academic Health [email protected] Science Centre, Division of Developmental Biology and Medicine Faculty of Biology, Medicine and Health, The University of Manchester. Project Manager GMEC SCN [email protected] Project Officer GMEC SCN [email protected] Endorsement Process Date of Presented for ratification at GMEC SCN Maternity Steering Group on:15th February ratification 2019 Application All Staff Circulation Issue Date: March 2019 Circulated by [email protected] Review Review Date: March 2021 Responsibility of: GMEC Maternity SCN Date placed on March 2019 the Intranet: Acknowledgements On behalf of the Greater Manchester and Eastern Cheshire and Strategic Clinical Networks, I would like to take this opportunity to thank the contributors for their enthusiasm, motivation and dedication in the development of these guidelines. Miss Karen Bancroft Maternity Clinical Lead for the Greater Manchester & Eastern Cheshire SCN GMEC RFM Guideline FINAL V1.3a 130619 Issue Date 15/02/2019 Version V1.3a Status Final Review Date Page 2 of 19 Contents 1 What is this Guideline for and Who should use it? ......................................................................... 4 2 What -

Parental Consent Form for a Minor Seeking Abortion
Parental Consent Form for a Minor Seeking Abortion Parental Statement: I certify that I, ___________________________, am the parent of _________________________________ (name of parent) (minor daughter name) and give consent for ____________________________ to perform an abortion on my daughter. I understand (physician name) that any person who knowingly makes a fraudulent statement in this regard commits a felony. Date: , 20 . Signature of Parent/Managing Conservator/Guardian I certify I have witnessed the execution of this consent by the parent. Subscribed and sworn to before me on this day of 20 (day) (month) Seal NOTARY PUBLIC in and for The State of OKLAHOMA My commission expires: Required attachments: - Copy of government-issued proof of identification - Written documentation that establishes that he or she is the lawful parent of the pregnant female Physician Statement: I, ____________________________, certify that according to my best information and belief, a reasonable person under (Physician name) similar circumstances would rely on the information presented by both the minor and her parent as sufficient evidence of identity. Date: , 20 . Signature of Physician ___________ (Parent Initials) Oklahoma State Department of Health 11/2013 Health Care Information Page 1 of 9 Consent of a Minor & Parental Consent Statement The law of the State of Oklahoma (Title 63, Section 1-740.13) requires physicians to obtain the consent of the minor and parent using this form prior to performing an abortion on a minor who is not emancipated. -
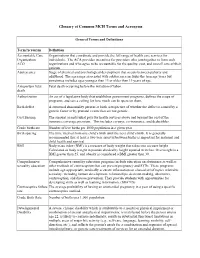
Glossary of Common MCH Terms and Acronyms
Glossary of Common MCH Terms and Acronyms General Terms and Definitions Term/Acronym Definition Accountable Care Organizations that coordinate and provide the full range of health care services for Organization individuals. The ACA provides incentives for providers who join together to form such ACO organizations and who agree to be accountable for the quality, cost, and overall care of their patients. Adolescence Stage of physical and psychological development that occurs between puberty and adulthood. The age range associated with adolescence includes the teen age years but sometimes includes ages younger than 13 or older than 19 years of age. Antepartum fetal Fetal death occurring before the initiation of labor. death Authorization An act of a legislative body that establishes government programs, defines the scope of programs, and sets a ceiling for how much can be spent on them. Birth defect A structural abnormality present at birth, irrespective of whether the defect is caused by a genetic factor or by prenatal events that are not genetic. Cost Sharing The amount an individual pays for health services above and beyond the cost of the insurance coverage premium. This includes co-pays, co-insurance, and deductibles. Crude birth rate Number of live births per 1000 population in a given year. Birth spacing The time interval from one child’s birth until the next child’s birth. It is generally recommended that at least a two-year interval between births is important for maternal and child health and survival. BMI Body mass index (BMI) is a measure of body weight that takes into account height. -

If You Are Pregnant: INFORMATION on FETAL DEVELOPMENT, ABORTION and ALTERNATIVES August 2019
If You Are Pregnant: INFORMATION ON FETAL DEVELOPMENT, ABORTION AND ALTERNATIVES August 2019 IF YOU ARE PREGNANT: INFORMATION ON FETAL DEVELOPMENT, ABORTION AND ALTERNATIVES If You Are Pregnant: Information on Fetal Development, Abortion and Alternatives Resources used by the Minnesota Department of Health for this publication are Human Embryology and Developmental Biology, Fifth Edition, 2014; Larsen’s Human Embryology, Fifth Edition, 2014; The Developing Human, 10th Edition, 2016; and In the Womb, 2006. The photographs in this booklet are credited to Lennart Nilsson/TT Images and are used by permission; except for week 38 copyright Minnesota Department of Health. The illustrations found throughout this booklet were created by Peg Gerrity, Houston, Texas. Copyright: http://www.peggerrity.com. Minnesota Department of Health Division of Child and Family Health PO Box 64882 St. Paul, MN 55164-0882 651-201-3580 Women's Right to Know (https://www.health.state.mn.us/people/wrtk/index.html) Upon request, this material will be made available in an alternative format such as large print, Braille or audio recording. Printed on recycled paper. 2 IF YOU ARE PREGNANT: INFORMATION ON FETAL DEVELOPMENT, ABORTION AND ALTERNATIVES Contents If You Are Pregnant: INFORMATION ON FETAL DEVELOPMENT, ABORTION AND ALTERNATIVES............................................................................................................................. 1 Introduction ............................................................................................................................... -

Prospective Study of Maternal Perception of Decreased Fetal Movement in Third Trimester and Evaluation of Its Correlation with Perinatal Compromise
International Journal of Reproduction, Contraception, Obstetrics and Gynecology Nandi N et al. Int J Reprod Contracept Obstet Gynecol. 2019 Feb;8(2):687-691 www.ijrcog.org pISSN 2320-1770 | eISSN 2320-1789 DOI: http://dx.doi.org/10.18203/2320-1770.ijrcog20190306 Original Research Article Prospective study of maternal perception of decreased fetal movement in third trimester and evaluation of its correlation with perinatal compromise Nupur Nandi, Ritika Agarwal* Department of Obstetrics and Gynecology, Teerthankar Mahaveer Medical College and Research Center, Moradabad, Uttar Pradesh, India Received: 14 November 2018 Accepted: 29 December 2018 *Correspondence: Dr. Ritika Agarwal, E-mail: [email protected] Copyright: © the author(s), publisher and licensee Medip Academy. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Background: Intrauterine fetal movements are sign of fetal life and well being. Perception of decreased fetal movements by the expecting mother is a common concern for both the mother and her obstetrician. Inadequate evaluation of reported decreased fetal movements may lead to catastrophic perinatal outcome. These necessitates us to identify the mothers perceiving decreased fetal movements, evaluating them to identify any risk factor, and follow up them to know the correlation with perinatal outcome. Methods: Antenatal mothers with singleton pregnancy at third trimester are recruited from OPD/ Emergency of Obstetrics and Gynaecology departments of Teerthankar Mahaveer Medical College and Research Center, Moradabad, Uttar Pradesh, India. Both case and control group comprise of 80 mothers matched by demographic profile, with perception of decreased fetal movements only in case group. -

An EPIC Predictor of Gestational Age and Its Application to Newborns Conceived by Assisted Reproductive Technologies Kristine L
Haftorn et al. Clin Epigenet (2021) 13:82 https://doi.org/10.1186/s13148-021-01055-z RESEARCH Open Access An EPIC predictor of gestational age and its application to newborns conceived by assisted reproductive technologies Kristine L. Haftorn1,2,3* , Yunsung Lee1,2, William R. P. Denault1,2,4, Christian M. Page2,5, Haakon E. Nustad2,6, Robert Lyle2,7, Håkon K. Gjessing2,4, Anni Malmberg8, Maria C. Magnus2,9,10, Øyvind Næss3,11, Darina Czamara12, Katri Räikkönen8, Jari Lahti8, Per Magnus2, Siri E. Håberg2, Astanand Jugessur1,2,4† and Jon Bohlin2,13† Abstract Background: Gestational age is a useful proxy for assessing developmental maturity, but correct estimation of gestational age is difcult using clinical measures. DNA methylation at birth has proven to be an accurate predictor of gestational age. Previous predictors of epigenetic gestational age were based on DNA methylation data from the Illumina HumanMethylation 27 K or 450 K array, which have subsequently been replaced by the Illumina Methylatio- nEPIC 850 K array (EPIC). Our aims here were to build an epigenetic gestational age clock specifc for the EPIC array and to evaluate its precision and accuracy using the embryo transfer date of newborns from the largest EPIC-derived dataset to date on assisted reproductive technologies (ART). Methods: We built an epigenetic gestational age clock using Lasso regression trained on 755 randomly selected non-ART newborns from the Norwegian Study of Assisted Reproductive Technologies (START)—a substudy of the Norwegian Mother, Father, and Child Cohort Study (MoBa). For the ART-conceived newborns, the START dataset had detailed information on the embryo transfer date and the specifc ART procedure used for conception. -

A B C Pregnancy Terms and Definitions
Pregnancy Terms and Definitions Obstetrics & Gynecology A After pains or afterbirth pains: Contractions of the uterus that occur after your baby is born, as the uterus returns to its normal size. This may cause cramping for a few days, especially if this is not your first baby or if you are nursing. Amniocentesis: the removal of a sample of amniotic fluid by means of a needle inserted through the mother’s abdominal wall; used for genetic and biochemical analysis of the baby. Amniotic fluid: the liquid surrounding and protecting the baby within the amniotic sac throughout pregnancy. Amniotic sac: the membrane within the uterus that contains the baby and the amniotic fluid. Analgesic: Medication that relieves or reduces pain. Anesthesia: Loss of feeling. There are three ways of doing this: general, local and epidural. Anesthesiologist: A doctor who specializes in the use of anesthesia. Anesthetist: A registered nurse who has special training in anesthesia. Apgar score rating: A system to evaluate the health of your baby immediately after birth. The score can be zero to 10, based on appearance and color, pulse, reflexes, activity and respiration. B Baby blues: A mild depression many women feel in the first few weeks after birth. Braxton-Hicks contractions: Mild, usually painless contractions that occur during the entire pregnancy, but are only felt from the 5th month on. Breech birth: Baby is born feet or buttocks first. C Cephalopelvic disproprition (CPD): Baby’s head is too large for the mother’s pelvic bones. Cervix: the neck of the uterus; Pap smears are taken from the cervix.