Ibdoc® Calprotectin Home Test
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Oneplus 3T Smartphone
gadgets corner SMartphONE ONEPLUS 3T — ashok Pandey ith the tagline of ‘Never Settle’ OnePlus revamped its previous smartphone OnePlus 3 and now it has launched 3T in the Wmarket. It’s an upgraded ‘Turbo’ version of OnePlus 3 which was an amazing device. Looks fab like its successor: There is no change in terms of build quality and design. 3T has an ergonomic solid design which gives it a premium look and comfortable feel in your hand. The back gets sandstone finish and Graphite in color with a bulging camera. The sides are slightly curved with the thin profile of 7.3mm only. The front is covered with glass, giving it premium look. At the bottom of the screen, one fingerprint sensor and two touch sensitive Price: `34,999/- navigation buttons are placed. The fingerprint unlocker and invisible backlit capacitive touch keys can be customized as per your need. AMOLED screen producing great colors: The elite body holds 5.5-inch Optic AMOLED Full HD screen with 1920 x 1080 pixels resolution. The new screen is better than the previous one with improved brightness, deep black levels, good contrast ratio, and color reproduction. You can easily operate the phone even in direct sunlight, additionally, there is a night mode to soothe your eyes and protect from strain. With great viewing angles, this lets you enjoy games and videos with you friends and family. 3T is still using 2-D curved Gorilla SCORE Glass 4 protection on the top of the screen. Price: 9/10 Awesome image quality: It boasts a 16 MP camera at the rear Performance: 10/10 Overall: (f/2.0 Aperture, 1.12 µm Pixel size.) with high-speed autofocus Features: 10/10 10/10 technology (PDAF), updated Electronic (EIS) and Optical (OIS) stabilization technologies and the lens is protected by sapphire glass. -

RADA Sense Mobile Application End-User Licence Agreement
RADA Sense Mobile Application End-User Licence Agreement PLEASE READ THESE LICENCE TERMS CAREFULLY BY CONTINUING TO USE THIS APP YOU AGREE TO THESE TERMS WHICH WILL BIND YOU. IF YOU DO NOT AGREE TO THESE TERMS, PLEASE IMMEDIATELY DISCONTINUE USING THIS APP. WHO WE ARE AND WHAT THIS AGREEMENT DOES We Kohler Mira Limited of Cromwell Road, Cheltenham, GL52 5EP license you to use: • Rada Sense mobile application software, the data supplied with the software, (App) and any updates or supplements to it. • The service you connect to via the App and the content we provide to you through it (Service). as permitted in these terms. YOUR PRIVACY Under data protection legislation, we are required to provide you with certain information about who we are, how we process your personal data and for what purposes and your rights in relation to your personal data and how to exercise them. This information is provided in https://www.radacontrols.com/en/privacy/ and it is important that you read that information. Please be aware that internet transmissions are never completely private or secure and that any message or information you send using the App or any Service may be read or intercepted by others, even if there is a special notice that a particular transmission is encrypted. APPLE APP STORE’S TERMS ALSO APPLY The ways in which you can use the App and Documentation may also be controlled by the Apple App Store’s rules and policies https://www.apple.com/uk/legal/internet-services/itunes/uk/terms.html and Apple App Store’s rules and policies will apply instead of these terms where there are differences between the two. -

America's Best Deserve Our Best
Teachers and their Families America’s Best Deserve our best 25% Discount for Eligible Educators Certified/licensed K-12 classroom teachers/educators are eligible Existing customers qualify and it is for ALL the lines on your plan No Purchase Necessary Bring your Own devices to AT&T & get up to $500 in pre-paid Visa cards! Call - Text - Email Additional Promotions Authorized AT&T Retailer JP Stork *FREE Devices 720-635-6119 *FREE Wireless Charging [email protected] Pads w /3+ new phones AT&T April Promotional Pricing *No purchase necessary for 25% discount Eligible Devices: • Eligible Purchased Smartphones o iPhone XS 64GB ($900), 256GB ($1050), 512GB ($1250) o iPhone XR 64GB ($500), 128GB ($550) o iPhone 11 Pro 64GB ($900), 256GB ($1050), 512GB ($1250) o iPhone 11 Pro Max 64GB ($1000), 256GB ($1150), 512GB ($1350) o iPhone 12 mini 64GB ($700), 128GB ($750), 256GB ($850) o iPhone 12 64GB ($800), 128GB ($850), 256GB ($950) o iPhone 12 Pro 128GB ($1000), 256GB ($1100), 512GB ($1300) o iPhone 12 Pro Max 128GB ($1100), 256GB ($1200), 512GB ($1400) • Eligible Trade-in Smartphones: o To qualify for $700 credit, minimum Trade-In value must be $95 or higher after device condition questions have been answered o Eligible devices: ▪ Apple: 8, 8 Plus, X, XR, XS, XS Max, 11, 11 Pro, 11 Pro Max, 12, 12 mini, 12 Pro, 12 Pro Max ▪ Samsung: A71, A71 5G, Fold, Z Fold2 5G, Galaxy S9, Galaxy S9+, Galaxy S9+ Duos, Galaxy S10, Galaxy S10+, Galaxy S10 5G, Galaxy S10e, Galaxy S10 Lite, Galaxy S20, Galaxy S20 Ultra 5G, Galaxy S20+ 5G, Note9, Note10, Note10+, -

Lista Device Compatibili.Xlsx
Android iOS (Cloud Anchors Only) iOS Acer Chromebook Tab 10 [1] iPhone XR iPhone XS ROG Phone iPhone XS and XS Max iPhone XS Max Zenfone 6 iPhone X iPhone XR Asus Zenfone AR iPhone 8 and 8 Plus iPhone X iPhone Zenfone ARES iPhone 7 and 7 Plus iPhone 8 General GM 9 Plus iPhone 6S and 6S Plus iPhone 8 Plus Mobile Nexus 5X [2] iPhone SE iPhone 7 Nexus 6P [3] Pixel, Pixel XL Google Pixel 2, Pixel 2 XL Pixel 3, Pixel 3 XL Pixel 3a, Pixel 3a XL Nokia 6 (2018) [4] Nokia 6.1 Plus Nokia 7 Plus HMD Nokia 7.1 Global Nokia 8 [5] Nokia 8 Sirocco Nokia 8.1 Honor 8X, Honor 10 Honor View 10 Lite Honor V20 Mate 20 Lite, Mate 20, Mate 20 Pro, Mate 20 X Nova 3, Nova 3i Huawei Nova 4 P20, P20 Pro P30, P30 Pro Porsche Design Mate RS, Porsche Design Mate 20 RS Y9 2019 G6 [6] G7 Fit, G7 One, G7 ThinQ [7] G8 ThinQ [8] Q6 Q8 LG V30, V30+, V30+ JOJO, LG Signature Edition 2017 [9] V35 ThinQ, LG Signature Edition 2018 [10] V40 ThinQ V50 ThinQ [11] Moto G5S Plus Moto G6, Moto G6 Plus Moto G7, Moto G7 Plus, Moto G7 Power, Moto G7 Play Motorola Moto One, Moto One Power Moto X4 [12] Moto Z2 Force [13] Moto Z3, Moto Z3 Play OnePlus 3T [14] OnePlus 5, OnePlus 5T OnePlus OnePlus 6, OnePlus 6T OnePlus 7, OnePlus 7 Pro, OnePlus 7 Pro 5G R17 Pro Oppo Reno 10x Zoom Galaxy A3 (2017) [15] Samsung Galaxy A5 (2017) Android iOS (Cloud Anchors Only) iOS Galaxy A6 (2018) Galaxy A7 (2017) Galaxy A8, Galaxy A8+ (2018) Galaxy A30, Galaxy A40, Galaxy A50, Galaxy A60, Galaxy A70, Galaxy A80 Galaxy J5 (2017), Galaxy J5 Pro [16] Galaxy J7 (2017), Galaxy J7 Pro [17] Galaxy Note8 Galaxy Note9 -
Oneplus 6T User Manual Index
OnePlus 6T User Manual Index 04 What’s in The Box? 24 Notch Display Settings 05 Device 25 OnePlus Fast Charging 06 Power On 26 OxygenOS 07 Setup Wizard 27 Launcher 08 Migrating Data (OnePlus Switch) 28 App Drawer 09 Insert SIM Card (SIM Card Tray) 29 Hidden Space 10 Screen Unlock 30 Notification Shade 11 Face Unlock 31 Shelf 12 Alert Slider 32 Gestures 13 Camera 33 App Long Press 14 Camera Interface 34 Reading Mode 15 Choosing Camera Modes and Settings 35 Gaming mode 16 Studio Lighting 36 Dialer 17 Nightscape 37 Messenger 18 Portrait Mode 38 Gallery 19 Video 39 Recorder 20 Video Editor 40 File Manager 21 Pro Mode 41 App Permission 22 Pro Mode - Continued 23 Optic AMOLED Display 2 Welcome Thank You! We believe in sharing the best technology, designed to be fast, smooth, and user-centric. The OnePlus 6T offers a fast and smooth experience, with an emphasis on speed. Navigating between apps, photos and games is now easier than it’s ever been. With speed at the center of its design, the OnePlus 6T now ensures that your time receives its optimum value. With our continued attention to improve swift and smooth transitions based on a sense of speed, the OnePlus 6T is our fastest product yet. A beautiful combination of sophisticated hardware and software allows you to experience speed like you’ve never done before. 3 What’s in The Box OnePlus 6T Screen Protector Translucent Case USB Type-C Cable Power Adapter SIM Tray Ejector Quick Start Guide USB Type-C 3.5mm (pre-applied) Safety Information Adapter 4 | What’s in the Box Device OxygenOS Device Alert Slider Nano SIM Slot Volume Power Sleep/Wake USB Type-C 5 | What’s in the Box Device OxygenOS Power On Turn on your OnePlus 6T by pressing and holding the <Power> button (found on the right side of the phone) for a few seconds. -
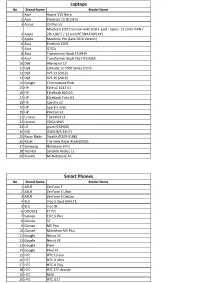
Type-C Compatible Device List 1.Xlsx
Laptops No Brand Name Model Name 1 Acer Aspire V15 Nitro 2 Acer Predator 15 (N15P3) 3 Aorus X3 Plus v5 MacBook (2015 version with USB-C port - Specs: 13.1mm THIN / 4 Apple 2lb. LIGHT / 12-inch RETINA DISPLAY) 5 Apple MacBook Pro (Late 2016 Version) 6 Asus EeeBook E202 7 Asus G752v 8 Asus Transformer Book T100HA 9 Asus Transformer Book Flip (TP200SA) 10 Dell Alienware 17 11 Dell Latitude 12 7000 Series (7275 12 Dell XPS 13 (2016) 13 Dell XPS 15 (2016) 14 Google Chromebook Pixel 15 HP Elite x2 1012 G1 16 HP EliteBook 840 G3 17 HP EliteBook Folio G1 18 HP Spectre x2 19 HP Spectre x360 20 HP Pavilion x2 21 Lenovo ThinkPad 13 22 Lenovo YOGA 900S 23 LG gram (15Z960) 24 MSI GS60 (MS-16H7) 25 Razer Blade Stealth (RZ09-0168) 26 Razer The New Razer Blade(2016) 27 Samsung Notebook 9 Pro 28 Toshiba Satellite Radius 12 29 Xiaomi Mi Notebook Air Smart Phones No Brand Name Model Name 1 ASUS ZenFone 3 2 ASUS ZenFone 3 Ultra 3 ASUS ZenFone 3 Deluxe 4 BLU Vivo 5 Dual SIM LTE 5 BLU Vivo XL 6 DOOGEE F7 Pro 7 Gionee Elife S Plus 8 Gionee S6 9 Gionee M5 Plus 10 Gionee Marathon M5 Plus 11 Google Nexus 5X 12 Google Nexus 6P 13 Google Pixel 14 Google Pixel XL 15 HTC HTC 10 evo 16 HTC HTC U Ultra 17 HTC HTC U Play 18 HTC HTC 10 Lifestyle 19 HTC M10 20 HTC HTC U11 21 Huawei Honor 22 Huawei Honor 8 23 Huawei Honor 9 24 Huawei Honor Magic 25 Huawei Hornor V9 26 Huawei Mate 9 27 Huawei Mate 9 Pro 28 Huawei Mate 9 Porsche Design 29 Huawei Nexus 6P 30 Huawei Nova2 31 Huawei P9 32 Huawei P10 33 Huawei P10 Plus 34 Huawei V8 35 Lenovo ZUK Z1(China) 36 Lenovo Zuk Z2 37 -

Barometer of Mobile Internet Connections in Indonesia Publication of March 14Th 2018
Barometer of mobile Internet connections in Indonesia Publication of March 14th 2018 Year 2017 nPerf is a trademark owned by nPerf SAS, 87 rue de Sèze 69006 LYON – France. Contents 1 Methodology ................................................................................................................................. 2 1.1 The panel ............................................................................................................................... 2 1.2 Speed and latency tests ....................................................................................................... 2 1.2.1 Objectives and operation of the speed and latency tests ............................................ 2 1.2.2 nPerf servers .................................................................................................................. 2 1.3 Tests Quality of Service (QoS) .............................................................................................. 2 1.3.1 The browsing test .......................................................................................................... 2 1.3.2 YouTube streaming test ................................................................................................ 3 1.4 Filtering of test results .......................................................................................................... 3 1.4.1 Filtering of devices ........................................................................................................ 3 2 Overall results 2G/3G/4G ............................................................................................................ -

Electronic 3D Models Catalogue (On July 26, 2019)
Electronic 3D models Catalogue (on July 26, 2019) Acer 001 Acer Iconia Tab A510 002 Acer Liquid Z5 003 Acer Liquid S2 Red 004 Acer Liquid S2 Black 005 Acer Iconia Tab A3 White 006 Acer Iconia Tab A1-810 White 007 Acer Iconia W4 008 Acer Liquid E3 Black 009 Acer Liquid E3 Silver 010 Acer Iconia B1-720 Iron Gray 011 Acer Iconia B1-720 Red 012 Acer Iconia B1-720 White 013 Acer Liquid Z3 Rock Black 014 Acer Liquid Z3 Classic White 015 Acer Iconia One 7 B1-730 Black 016 Acer Iconia One 7 B1-730 Red 017 Acer Iconia One 7 B1-730 Yellow 018 Acer Iconia One 7 B1-730 Green 019 Acer Iconia One 7 B1-730 Pink 020 Acer Iconia One 7 B1-730 Orange 021 Acer Iconia One 7 B1-730 Purple 022 Acer Iconia One 7 B1-730 White 023 Acer Iconia One 7 B1-730 Blue 024 Acer Iconia One 7 B1-730 Cyan 025 Acer Aspire Switch 10 026 Acer Iconia Tab A1-810 Red 027 Acer Iconia Tab A1-810 Black 028 Acer Iconia A1-830 White 029 Acer Liquid Z4 White 030 Acer Liquid Z4 Black 031 Acer Liquid Z200 Essential White 032 Acer Liquid Z200 Titanium Black 033 Acer Liquid Z200 Fragrant Pink 034 Acer Liquid Z200 Sky Blue 035 Acer Liquid Z200 Sunshine Yellow 036 Acer Liquid Jade Black 037 Acer Liquid Jade Green 038 Acer Liquid Jade White 039 Acer Liquid Z500 Sandy Silver 040 Acer Liquid Z500 Aquamarine Green 041 Acer Liquid Z500 Titanium Black 042 Acer Iconia Tab 7 (A1-713) 043 Acer Iconia Tab 7 (A1-713HD) 044 Acer Liquid E700 Burgundy Red 045 Acer Liquid E700 Titan Black 046 Acer Iconia Tab 8 047 Acer Liquid X1 Graphite Black 048 Acer Liquid X1 Wine Red 049 Acer Iconia Tab 8 W 050 Acer -

Reviewer's Guide Booklets
Reviewer’s Guide Meet the OnePlus 5T — At OnePlus, we are constantly working to improve the user With the OnePlus 5T, you get: experience. We strive to continuously improve our products with • 6.01” Full Optic AMOLED Display with an 18:9 Aspect Ratio every generation. Because OnePlus users are some of the most discerning and tech-savvy in the world, we wanted to deliver these • High resolution dual camera system, with a 16 MP main key improvements through an upgraded device to provide the camera supported by a 20 MP secondary camera for enhanced best user experience today. low-light performance and beautiful portraits We have added features, engineered optimizations and made • Advanced Facial Recognition strides in hardware that could not be delivered via software updates - all based on feedback from our community, and our own • Dash Charge, which gives you a day’s power in half an hour sense of what we felt would make the phone better overall. • Rear mounted ceramic fingerprint sensor that unlocks your This approach has guided the development and refinement of our phone in under 0.2 seconds most complete and competitive product ever – the OnePlus 5T. • Octa-core Qualcomm® Snapdragon™ 835 processor (up to 2.45 GHz) • Up to 8 GB of RAM and 128 GB of Storage • A refined Android experience with OxygenOS 4.7 • OnePlus’ unique Alert Slider, which allows you to effortlessly switch between three notification profiles: Silent, Do Not Disturb and Ring 2 3 Design — The seamless aluminum unibody of the OnePlus 5T is both functionally and visually slim, creating a phone that is not only comfortable to hold but exceedingly durable. -

In the United States District Court for the Eastern District of Texas Marshall Division
Case 2:19-cv-00057-JRG Document 1 Filed 02/15/19 Page 1 of 33 PageID #: 1 IN THE UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF TEXAS MARSHALL DIVISION EVS CODEC TECHNOLOGIES, LLC and § SAINT LAWRENCE COMMUNICATIONS, § LLC, § Case No. 2:19-cv-00057 § Plaintiffss, § § JURY TRIAL DEMANDED v. § § ONEPLUS TECHNOLOGY (SHENZHEN) § CO., LTD. and T-MOBILE USA, INC., § § Defendants. § COMPLAINT FOR PATENT INFRINGEMENT Case 2:19-cv-00057-JRG Document 1 Filed 02/15/19 Page 2 of 33 PageID #: 2 EVS Codec Technologies, LLC (“ECT”) and Saint Lawrence Communications, LLC (“SLC”) (collectively “Plaintiffs”) hereby submit this Complaint for patent infringement against Defendants OnePlus Technology (Shenzhen) Co., Ltd. (“OnePlus”) and T-Mobile USA, Inc. (“T- Mobile”) (collectively “Defendants”) and state as follows: THE PARTIES 1. ECT is a Texas limited liability company with a principal place of business at 2323 S. Shepherd, 14th floor, Houston, Texas 77019-7024. 2. SLC is a Texas limited liability company, having a principal place of business at 6136 Frisco Square Blvd., Suite 400, Frisco, Texas 75034. 3. On information and belief, Defendant T-Mobile USA, Inc. is a Washington corporation having a regular and established principal place of business at 12920 SE 38th Street, Bellevue, Washington 98006. T-Mobile USA, Inc. may be served through its registered agent, Corporation Service Company, at 211 E. 7th Street, Suite 620, Austin, Texas 78701. 4. On information and belief, Defendant OnePlus is a Chinese corporation having a principal place of business at 18F Tairan Building, Block C, Tairan 8th Road, Chegongmiao, Futian District, Shenzhen, Guangdong 518040, China. -

Brand Old Device
# New Device Old Device - Brand Old Device - Model Name 1 Galaxy A6+ Asus Asus Zenfone 2 Laser ZE500KL 2 Galaxy A6+ Asus Asus Zenfone 2 Laser ZE601KL 3 Galaxy A6+ Asus Asus ZenFone 2 ZE550ML 4 Galaxy A6+ Asus Asus Zenfone 2 ZE551ML 5 Galaxy A6+ Asus Asus Zenfone 3 Laser 6 Galaxy A6+ Asus Asus Zenfone 3 Max ZC520TL 7 Galaxy A6+ Asus Asus Zenfone 3 Max ZC553KL 8 Galaxy A6+ Asus Asus Zenfone 3 ZE520KL 9 Galaxy A6+ Asus Asus Zenfone 3 ZE552KL 10 Galaxy A6+ Asus Asus Zenfone 3s Max 11 Galaxy A6+ Asus Asus Zenfone Max 12 Galaxy A6+ Asus Asus Zenfone Selfie 13 Galaxy A6+ Asus Asus ZenFone Zoom ZX550 14 Galaxy A6+ Gionee Gionee A1 15 Galaxy A6+ Gionee Gionee A1 Lite 16 Galaxy A6+ Gionee Gionee A1 Plus 17 Galaxy A6+ Gionee Gionee Elife E8 18 Galaxy A6+ Gionee Gionee Elife S Plus 19 Galaxy A6+ Gionee Gionee Elife S7 20 Galaxy A6+ Gionee Gionee F103 21 Galaxy A6+ Gionee Gionee F103 Pro 22 Galaxy A6+ Gionee Gionee Marathon M4 23 Galaxy A6+ Gionee Gionee Marathon M5 24 Galaxy A6+ Gionee Gionee marathon M5 Lite 25 Galaxy A6+ Gionee Gionee Marathon M5 Plus 26 Galaxy A6+ Gionee Gionee P5L 27 Galaxy A6+ Gionee Gionee P7 Max 28 Galaxy A6+ Gionee Gionee S6 29 Galaxy A6+ Gionee Gionee S6 Pro 30 Galaxy A6+ Gionee Gionee S6s 31 Galaxy A6+ Gionee Gionee X1s 32 Galaxy A6+ Google Google Pixel 33 Galaxy A6+ Google Google Pixel XL LTE 34 Galaxy A6+ Google Nexus 5X 35 Galaxy A6+ Google Nexus 6 36 Galaxy A6+ Google Nexus 6P 37 Galaxy A6+ HTC Htc 10 38 Galaxy A6+ HTC Htc Desire 10 Pro 39 Galaxy A6+ HTC Htc Desire 628 40 Galaxy A6+ HTC HTC Desire 630 41 Galaxy A6+ -

Case 2:20-Cv-00385 Document 1 Filed 12/15/20 Page 1 of 26 Pageid #: 1
Case 2:20-cv-00385 Document 1 Filed 12/15/20 Page 1 of 26 PageID #: 1 IN THE UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF TEXAS MARSHALL DIVISION § NORTHSTAR SYSTEMS LLC, § § Case No. Plaintiff, § § JURY TRIAL DEMANDED v. § § SHENZHEN ONEPLUS SCIENCE & § TECHNOLOGY CO., LTD., § § Defendant § COMPLAINT FOR PATENT INFRINGEMENT Plaintiff NorthStar Systems LLC (“NorthStar” or “Plaintiff”) for its Complaint against Defendant Shenzhen OnePlus Science & Technology Co., Ltd. (“OnePlus” or “Defendant”) alleges as follows: THE PARTIES 1. NorthStar is a limited liability company organized and existing under the laws of the State of Texas, with its principal place of business located at 203 East Travis Street, Marshall, Texas 75670 2. Upon information and belief, Defendant OnePlus is a corporation organized and existing under the laws of China, with its principal place of business located at 18F, Tairan Building, Block C, Tairan 8th Road, Chegongmiao, Futian District, Shenzhen, Guangdong 518040, China, and may be served pursuant to the provisions of the Hague Convention. OnePlus is a leading manufacturer and seller of smartphones in the world and in the United States. Upon information and belief, OnePlus does business in Texas and in the Eastern District of Texas, directly or through its subsidiaries. Case 2:20-cv-00385 Document 1 Filed 12/15/20 Page 2 of 26 PageID #: 2 JURISDICTION 3. This is an action for patent infringement arising under the patent laws of the United States, 35 U.S.C. §§ 1, et seq. This Court has jurisdiction over this action pursuant to 28 U.S.C. §§ 1331 and 1338(a).