Chemistry Department 2015-16
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Table of Contents (PDF)
January 12, 2016 u vol. 113 u no. 2 From the Cover E249 Drought impacts on California forests E172 Kinases and prostate cancer metastasis E229 Potassium channel and sour taste 262 Tuning protein reduction potential 380 Lipid target of autoreactive T cells Contents THIS WEEK IN PNAS Cover image: Pictured is a 3D image of 237 In This Issue the canopy water content (CWC) of trees in the Jasper Ridge Biological Preserve at Stanford University, with low and high LETTERS (ONLINE ONLY) water content shown in red and blue, respectively. Gregory P. Asner et al. E105 Is nitric oxide important for the diastolic phase of the lymphatic found that CWC decreased measurably contraction/relaxation cycle? for an estimated 10.6 million hectares of Michael J. Davis forest, containing up to 888 million large E106 Reply to Davis: Nitric oxide regulates lymphatic contractions trees, between 2011 and 2015, with 1 Christian Kunert, James W. Baish, Shan Liao, Timothy P. Padera, and Lance L. Munn million hectares experiencing greater E107 Lion populations may be declining in Africa but not as Bauer et al. suggest than 30% CWC loss. The results suggest Jason Riggio, Tim Caro, Luke Dollar, Sarah M. Durant, Andrew P. Jacobson, Christian Kiffner, that if drought conditions in California Stuart L. Pimm, and Rudi J. van Aarde continue, forests might undergo E109 Reply to Riggio et al.: Ongoing lion declines across most of Africa warrant substantial changes. See the article by urgent action Asner et al. on pages E249–E255. Image Hans Bauer, Guillaume Chapron, Kristin Nowell, Philipp Henschel, Paul Funston, Luke T. -

1. AC Michael, Christian Activist for Human Rights
Endorsed by - (In alphabetical order) 1. A C Michael, Christian Activist For Human Rights And Former Member Delhi Minorities Commission 2. A. Hasan, Retired Banker 3. A. K Singh, NA 4. A. Reyna Shruti, Student 5. A. Giridhar Rao, NA 6. A. M. Roshan, Concerned Citizen 7. A. Selvaraj, Former Chief Commissioner Of Income Tax 8. Aakanksha, Student 9. Aakash Gautam, NA 10. Aakshi Sinha, NA 11. Aastha, Student/Teacher 12. Abde Mannaan Yusuf, Moderator IAD 13. Abdul Ghaffar, Manager, Private Firm 14. Abdul Kalam, NA 15. Abdul Mabood, Citizen 16. Abdul Wahab, Social Activist / Business 17. Abha Choudhuri, Homemaker And Caregiver 18. Abha Dev Habib, Assistant Professor, Miranda House, DU 19. Abha Rani Devi, NA 20. Abha, Research Scholar 21. Abhay Kardeguddi, CEO 22. Abhay, Lawyer 23. Abhijit Kundu, Faculty, DU 24. Abhijit Sinha, Mediaman 25. Abhinandan Sinha, NA 26. Achin Chakraborty, NA 27. Achla Sawhney, NA 28. Adithi, Teacher 29. Aditi Mehta, IAS Retd. 30. Aditya Mukherjee, Professor 31. Aditya Nigam, Academic, Delhi 32. Admiral L Ramdas, Former Chief Of Naval Staff 33. Adnan Jamal, Student 34. Adv. Ansar Indori, Human Rights Lawyer 35. Afaq Ullah, Social Worker 36. Aftab, Advocate 37. Agrima, Student 38. Ahmar Raza, Retired Scientist 39. Aiman Khan, Researcher 40. Aiman Siddiqui, Journalist 41. Aishah Kotecha, Principal 42. Aishwarya Bajpai, Student 43. Aishwarya, NA 44. Ajay Singh Mehta, NA 45. Ajay Skaria, Professor, History And Global Studies, University Of Minnesota 46. Ajay T G, Filmmaker 47. Ajin K Thomas, Researcher, Ahmedabad 48. Ajmal V, Freelance Journalist 49. Akash Bhatnagar, IT 50. Akha, NA 51. -

M.A. Political Science First Admission List
University of Delhi First Admission List - M.A. Political Science Page 1 of 19 Category : UNRESERVED (Entrance Based) Note : In case of the tie at the last seat(s) the information of the candidates are displayed at the end of this admission list. Entrance Exam. S.No. Roll No. Form No. Name Alloted Department/College Marks 1 13817908 17POLI1089423 DEEPAK KUMAR SINGH Ramjas College 263 2 13813355 17POLI1057124 KUMAR SAMANJASHYA Hindu College 260 3 13815687 17POLI1080560 NAVROZ SINGH Lady Shri Ram College for Women 258 4 13817843 17POLI1056365 KRITI TRIPATHI Hindu College 250 5 13810911 17POLI1042755 ISHA ANAND Hindu College 250 6 13814014 17POLI1010096 TUSHAR SABNANI Hindu College 249 7 13811831 17POLI1053553 SHASHANK SHEKHAR Hindu College 248 8 13814837 17POLI1027400 NITIN KUMAR KAMBOJ Kirori Mal College 248 9 13813416 17POLI1004793 MEDHA SHARMA Daulat Ram College 247 10 13814139 17POLI1007466 AKHILESH SINGH RANA Hindu College 247 11 13816925 17POLI1103032 TRISHA HARI Lady Shri Ram College for Women 245 12 13811502 17POLI1076474 SACHIN SRIVASTAVA Kirori Mal College 242 13 13810823 17POLI1059781 ANKIT MALIK Kirori Mal College 235 14 13810988 17POLI1129223 ASHISH ANAND Kirori Mal College 235 15 13814696 17POLI1005177 MRINALINI KUMAR Daulat Ram College 233 16 13814576 17POLI1063749 RAJKUMAR JACKSON SINGH Ramjas College 232 17 13815338 17POLI1014736 AMIT KUMAR SHARMA Kirori Mal College 228 18 13817589 17POLI1040583 PINGAKSCHYA PATTANAYAK Ramjas College 228 19 13815356 17POLI1042049 PRAJAPATHY R. Ramjas College 228 20 13810361 17POLI1019971 -

DNA Repair Systems Guardians of the Genome
GENERAL ARTICLE DNA Repair Systems Guardians of the Genome D N Rao and Yedu Prasad The 2015 Nobel Prize in Chemistry was awarded jointly to Tomas Lindahl, Paul Modrich and Aziz Sancar to honour their accomplishments in the field of DNA repair. Ever since the discovery of DNA structure and their importance in the storage of genetic information, questions about their stability became pertinent. A molecule which is crucial for the de- velopment and propagation of an organism must be closely D N Rao is a professor at the monitored so that the genetic information is not corrupted. Department of Biochemistry, Thanks to the pioneering research work of Lindahl, Sancar, Indian Institute of Science, Modrich and their colleagues, we now have an holistic aware- Bengaluru. His research ness of how DNA damage occurs and how the damage is rec- work primarily focuses on DNA interacting proteins in tified in bacteria as well as in higher organisms including hu- prokaryotes. This includes man beings. A comprehensive understanding of DNA repair restriction-modification has proven crucial in the fight against cancer and other debil- systems, DNA repair proteins itating diseases. from pathogenic bacteria and and proteins involved in The genetic information that guides the development, metabolism horizontal gene transfer and and reproduction of all living organisms and many viruses resides DNA recombination. in the DNA. This biological information is stored in the DNA Yedu Prasad is a graduate molecule as combinations of sequences that are formed by purine student working on his PhD and pyrimidine bases attached to the deoxyribose sugar (Figure under the guidance of D N 1). -
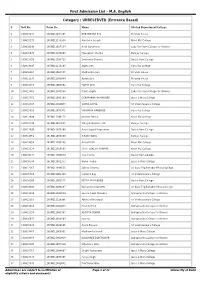
First Admission List - M.A
First Admission List - M.A. English Category : UNRESERVED (Entrance Based) # Roll No. Form No. Name Alloted Department/College 1 160815834 16ENGL1044205 DEBANGANA PAL Miranda House 2 160815171 16ENGL1133358 Alex John Joseph Kirori Mal College 3 160810398 16ENGL1017134 Avali Gandharva Lady Shri Ram College for Women 4 160815671 16ENGL1073063 Meenakshi Sharma Ramjas College 5 160813209 16ENGL1028713 Swarnima Dharwal Daulat Ram College 6 160814895 16ENGL1113183 Arpita Sen Hans Raj College 7 160816164 16ENGL1022555 Madhurima Sen Miranda House 8 160811195 16ENGL1081884 Asmita Jain Miranda House 9 160812459 16ENGL1009862 PARTH DUA Hans Raj College 10 160813863 16ENGL1075598 Prachi Gupta Lady Shri Ram College for Women 11 160815763 16ENGL1033269 DEBAPARNA MUKHERJEE Jesus & Mary College 12 160812335 16ENGL1099053 VANYA GOYAL Sri Venkateswara College 13 160815919 16ENGL1075971 PARAMIKA BANERJEE Hans Raj College 14 160812690 16ENGL1106175 Jasmine Bhalla Kirori Mal College 15 160813799 16ENGL1027835 Vinayak Gaurav Pant Ramjas College 16 160811698 16ENGL1073268 Avani Jayant Udgaonkar Daulat Ram College 17 160813961 16ENGL1039290 KIRAN YADAV Ramjas College 18 160814958 16ENGL1095185 Anibal Goth Kirori Mal College 19 160812174 16ENGL1050583 STUTI LOKESH SHARMA Kirori Mal College 20 160810271 16ENGL1093659 riya mishra Daulat Ram College 21 160810588 16ENGL1031232 Richa Thakur Jesus & Mary College 22 160812261 16ENGL1098555 Sohina Sharma Sri Guru Teg Bahadur Khalsa College 23 160815918 16ENGL1004389 Supratik Ray Sri Venkateswara College 24 160816208 -

N E W S I N B R I
N E W S I N B R I E F NOBEL PRIZE IN MEDICINE The Nobel Prize in Chemistry 2015 was awarded jointly to Tomas Lindahl, Paul Modrich and Aziz Sancar for elucidating At the height of the Vietnamese war, the common enemy of three different mechanisms by which errors occur in our DNA soldiers on either side of the battle lines was chloroquine- and the various mechanisms of DNA repair. In the 1970’s, resistant malaria. In 1964, the North Vietnamese Government Lindahl discovered that DNA undergoes regular decay and approached the Chinese leader Mao Tse Tung to find a damage. For example, cytosine loses an amino group to become solution to this deadly scourge. Mao immediately established uracil resulting in a mutation. Subsequently, he discovered an a military mission Project 523 with a main aim to discover a enzyme system called uracil-DNA glycosylase which corrects drug for resistant malaria. Youyou Tu was a phytochemist who this error. This process was called base excision repair. Aziz was in charge of the project. She and her team combed through Sancar discovered and cloned the gene for an enzyme called hundreds of old Chinese traditional medicine texts. Around photolyase which is critical in correcting DNA mutations caused 2000 chemicals extracted from various plants were evaluated. by high doses of UV exposure. He further went on to identify the Finally, they zeroed on to an extract of Artemesia annua L. exact chemical processes involved in this nucleotide excision also called Quinhao which was found to have excellent repair. Paul Modrich discovered the mechanism by which DNA antimalarial efficacy in a mouse model. -

Over 300 Academicians, Activists, Artists and Writers Condemn the State Violence and Unlawful Detention of Facul
Over 300 academicians, activists, artists and writers condemn the state violence and unlawful detention of faculty and student protesters of the University of Hyderabad We, academicians, activists, artists and writers, condemn the ongoing brutal attacks on and unlawful detention of peacefully protesting faculty and students at the University of Hyderabad by the University administration and the police. We also condemn the restriction of access to basic necessities such as water and food on campus. The students and faculty members of the University of Hyderabad were protesting the reinstatement of Dr. Appa Rao Podile as the ViceChancellor despite the ongoing judicial enquiry against him related to the circumstances leading to the death of the dalit student th Rohith Vemula on January 17 , 2016. Students and faculty members of the university community are concerned that this may provide him the opportunity to tamper with evidence and to influence witnesses. Suicides by dalit students have been recurring in the University of Hyderabad and other campuses across the country. The issue spiraled into a nationwide students’ protest with the death of the dalit scholar Rohith Vemula. The protests have pushed into the foreground public discussion and debate on the persistence of castebased discrimination in educational institutions, and surveillance and suppression of dissent and intellectual debate in university spaces. Since the morning of March 22 when Dr. Appa Rao returned to campus, the students and staff have been in a siegelike situation. The peacefully protesting staff and students were brutally lathicharged by the police, and 27 people were taken into custody. -

M.Sc. Chemistry First Admission List
University of Delhi First Admission List - M.Sc. Chemistry-2019 25-07-2019 09:14:51 University of Delhi Admission Category: Unreserved (Merit Based) Alloted Qualifying Category Filled # Form No. Name Department/College Marks %/GP Choice by Candidate 1 19CHEM1042438 NICY Hans Raj College 94.35 2 Unreserved 2 19CHEM1080777 VAISHNAVI RANA Hindu College 93.58 2 Unreserved 3 19CHEM1023488 NAVONEEL SEN Hindu College 93.32 2 Unreserved 4 19CHEM1080003 PRIYANKA JAIN Miranda House 91.41 2 Unreserved 5 19CHEM1096038 ACHYUT RANJAN Hindu College 91.15 1 Unreserved GOGOI 6 19CHEM1045437 PRIYANKA Miranda House 91.15 1 Unreserved 7 19CHEM1078611 POORVI ALLAWADI Hindu College 91.15 1 Unreserved 8 19CHEM1108812 MOHIT KUMAR Hindu College 90.63 1 Unreserved 9 19CHEM1020904 SOPHIA Hindu College 90.38 1 Unreserved 10 19CHEM1088703 PAYAL RANI Hindu College 90.25 1 Unreserved 11 19CHEM1034588 MANPREET KAUR Hindu College 89.99 1 Unreserved 12 19CHEM1039285 SAPNA Miranda House 89.40 1 OBC Non-Creamy layer 13 19CHEM1072493 BHARTI GAUBA Miranda House 89.34 1 Unreserved 14 19CHEM1021268 VASU MALHOTRA Hindu College 89.22 1 Unreserved 15 19CHEM1039776 ANMOL ANDOTRA Kirori Mal College 89.22 3 Unreserved 16 19CHEM1091552 SHIVANGI SHARMA Miranda House 88.83 2 Unreserved 17 19CHEM1078618 NIDHI Hans Raj College 88.68 3 Unreserved 18 19CHEM1034585 KRITIKA SHARMA Sri Guru Teg Bahadur Khalsa 88.57 2 Unreserved College 19 19CHEM1088137 YASHIKA SHARMA Miranda House 88.19 1 Unreserved 20 19CHEM1018001 ANUJ SHARMA Hans Raj College 87.94 1 Unreserved 21 19CHEM1030088 HIMANI Miranda House 87.80 1 Unreserved 22 19CHEM1016608 MERIN DAS Miranda House 87.80 2 Unreserved 23 19CHEM1080117 ASHA PANDEY Miranda House 87.68 1 Unreserved DISCLAIMER: Qualifying Examination Marks, given in this Admission List, are provided by the applicants. -

The Annual Report Submitted by IQAC Annual Quality Assurance Report 2015 -2016
The Annual Report Submitted by IQAC Annual Quality Assurance Report 2015 -2016 The IQAC at Aditi Mahavidyalaya undertook various engagement and developmental activities in pursuance of its all round excellence in the institution. INFRASTRUCTURAL IMPROVEMENTS Owing to the growing strengths of students a new block of storeys was constructed inside the college which gave us six new rooms ,a big hall for meetings and conferences and a new reading room and a new library room. One was turned to a medical room with basic medicines‟ a bed and a first aid facilities and inaugurated in October 1st,2015 by the honourable vice chancellor,University of Delhi. This year also saw a major renovation of the college hall, which received a false ceiling a new stage floor and a newcurtains. College has developed a multifaceted computer center for the campus users. The software and hardware is constantly upgraded to keep abreast with latest technology. The computer center has about 120 computers with latest special purpose software packages to meet the requirement of the curriculum, Wi-Fi Connectivity in the College, LAN connection on all the terminals, Internet facility on each terminal, 6 Laser Jet Printer to have the hard-copy of searched material from Internet or work- done in the center. The computer committee on the request of commerce department installed Tally ERP 9 in 40 systems for development of students and faculty. Event wise reports and photographs are regularly updated. Notices and information for upcoming programmes are uploaded on the college website. Refresher Programme Attended by Faculty: Dr Ritu Sharma Completed UGC sponsored Refresher Course - Winter school on „Folklore, Culture and Tradition‟ from CPDHE, University of Delhi from February 18th to March 9th , 2016. -

Curriculum Vitae
CURRICULUM VITAE Name Dr. Ritu Gupta Designation ASSISTANT PROFESSOR Address Department of Chemistry Daulat Ram College, University of Delhi Residence Address: B-2/21 First Floor Vasant Vihar new Delhi Residence Mobile Email [email protected] Web-Page Educational Qualifications Degree Institution Year Ph.D. University of Delhi 2001 M.Sc Chemistry Hindu College, University of Delhi 1996-97 B.Sc Chemistry Hindu College, University of Delhi 1994-95 Career Profile Worked as an Ad-hoc Lecturer in DDUC, University of Delhi from August 2000 to 2002. Worked as an Ad-hoc lecturer in Department of Chemistry, University of Delhi from August 2002 to April 2004. Worked as an Ad-hoc Lecturer in Department of Chemistry, University of Delhi from August 2002 to April 2004. Worked as an Ad-hoc Lecturer in Hansraj College, University of Delhi from August 2004 to August 2005. Assistant professor in the Chemistry department of Daulat Ram College, University of Delhi since July, 2005. Administrative Assignments Worked as Deputy Superintendent for Chemistry Practical Exam in the year 2010-2011. Worked as Teacher In charge of Chemistry Deptt in year 2012-13. Junior teacher representative member of Governing Body (2011-12). Co-Ordinator of Foundation Course / Applied Course (FYUP) evaluation work in Daulat Ram College (Dec 2013). Areas of Interest / Specialization Physical Chemistry Subjects Taught Chemistry, science and life, Environmental studies Research Guidance www.du.ac.in Page 1 1. Supervised a group of ten undergraduate students for innovation project DR-305: “Green method of defluoridation and estimation of fluoride ion from ground water and various other samples” in the year 2015. -
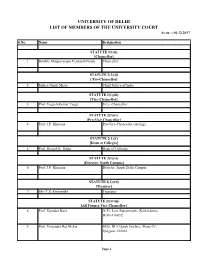
UNIVERSITY of DELHI LIST of MEMBERS of the UNIVERSITY COURT As on :- 04.12.2017
UNIVERSITY OF DELHI LIST OF MEMBERS OF THE UNIVERSITY COURT As on :- 04.12.2017 S.No. Name Designation STATUTE 2(1)(i) [Chancellor] 1 Hon'ble Muppavarapu Venkaiah Naidu Chancellor STATUTE 2(1)(ii) [ Pro-Chancellor] 2 Justice Dipak Misra Chief Justice of India STATUTE 2(1)(iii) [Vice-Chancellor] 3 Prof. Yogesh Kumar Tyagi Vice -Chancellor STATUTE 2(1)(iv) [Pro-Vice-Chancellor] 4 Prof. J.P. Khurana Pro-Vice-Chancellor (Acting) STATUTE 2(1)(v) [Dean of Colleges] 5 Prof. Devesh K. Sinha Dean of Colleges STATUTE 2(1)(vi) [Director, South Campus] 6 Prof. J.P. Khurana Director, South Delhi Campus STATUTE 2(1)(vii) [Tresurer] 7 Shri T.S. Kripanidhi Treasurer STATUTE 2(1)(viii) [All Former Vice-Chancellor] 8 Prof. Upendra Baxi A-51, Law Appartments, Karkardoma, Delhi-110092 9 Prof. Vrajendra Raj Mehta 5928, DLF Qutab Enclave, Phase-IV, Gurgaon-122002 Page 1 10 Prof. Deepak Nayyar 5-B, Friends Colony (West), New Delhi-110065 11 Prof. Deepak Pental Q.No. 7, Ty.V-B, South Campus, New Delhi-110021 12 Prof. Dinesh Singh 32, Chhatra Marg, University of Delhi, Delhi-110007 STATUTE 2(1)(ix) [Librarian] 13 Dr. D.V. Singh Librarian STATUTE 2(1)(x) [Proctor] 14 Prof. Neeta Sehgal Proctor (Offtg.) STATUTE 2(1)(xi) [Dean Student's Welfare] 15 Prof. Rajesh Tondon Dean Student's Welfare STATUTE 2(1)(xii) [Head of Departments] 16 Prof. Christel Rashmi Devadawson The Head Department of English University of Delhi Delhi-110007 17 Prof. Sharda Sharma The Head Department of Sanskrit University of Delhi Delhi-110007 18 Prof. -

Arijit Mcaf DEPARTMENT of PHYSICS & ASTROPHYSICS
Arijit Mcaf DEPARTMENT OF PHYSICS & ASTROPHYSICS University of Delhi Notice: For all the candidates in Category I, II and V, who do not have NET, colleges should verify their eligibility according to University regulations. LIST OF THE CANDIDATES FOR CONSIDERATION OF APPOINTMENT AS AD-HOC LECTURER IN PHYSICS IN DELHI UNIVERSITY COLLEGES- October 2020 *All the students who have been admitted to the Ph.D., programme in this Department under the new ordinance and who have not completed two years after registration, are advised that they will not be granted leave from Ph.D., to take up ad- hoc teaching assignment. In the event that such a student takes up an ad-hoc position, the Ph.D., registration is liable to be cancelled. Remarks: Candidates who are pursuing Ph.D., course and NET Qualified should have prior permission from their supervisor before appearing for the interview for Adhoc appointment in various colleges of the University. GENERAL Candidates: CATEGORY-I: First division from Graduation onwards + Ph.D., AWARDED S.No. Name/Address/Telephone No. Qualification Remarks 1. Aarti Tewari B.Sc., University of Delhi, 74.10%, H4-102, Mahaveer Enclave, Palam, New 2007 Delhi-110045` M.Sc., -do-, 63.70%, 2009 Ph:- 9910757461 Ph.D.,-DTU-2017 E-mail:- [email protected] 2. Abhishek Dwivedi B.Sc.(H), BHU, 60.39%, 2010 Vill & Post- Kajha, Distt- Mau, UP-276129 M.Sc., -do-, 72.60%, 2012 Ph:- 7897760331 Ph.D., BHU, 2017 E-mail:- [email protected] NET- LS-2012 3. Abhishek Kumar Singh B.Sc.(H), University of Delhi, 60%, 110, IInd Floor, Pocket-5, Sec.-22, Rohini, 2004 Delhi-110086 M.Sc., -do-, 60%, 2008 Ph:- 9990030872 Ph.D.,- University of Delhi -2014 E-mail:- [email protected] NET-JRF-2008 4.