Your 2021 BCBSVT Formulary Effective July 1, 2021
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Emergency Use Authorization (EUA) for Sotrovimab 500 Mg Center for Drug Evaluation and Research (CDER) Review
Emergency Use Authorization (EUA) for Sotrovimab 500 mg Center for Drug Evaluation and Research (CDER) Review Identifying Information Application Type EUA (EUA or Pre-EUA) If EUA, designate whether pre-event or intra-event EUA request. EUA Application EUA 000100 Number(s) Sponsor (entity EUA Sponsor requesting EUA or GlaxoSmithKline Research & Development Limited pre-EUA 980 Great West Road consideration), point Brentford Middlesex, TW8 9GS of contact, address, UK phone number, fax number, email GSK US Point of Contact address Debra H. Lake, M.S. Sr. Director Global Regulatory Affairs GlaxoSmithKline 5 Moore Drive PO Box 13398 Research Triangle Park, NC 27709-3398 (b) (6) Email: Phone Manufacturer, if GlaxoSmithKline, Parma. different from Sponsor Submission Date(s) Part 1: March 24, 2021 Part 2: March 29, 2021 Receipt Date(s) Part 1: March 24, 2021 Part 2: March 29, 2021 OND Division / Office Division of Antivirals /Office of Infectious Disease 1 Reference ID: 4802027 Product in the No Strategic National Stockpile (SNS) Distributor, if other (b) (4) than Sponsor I. EUA Determination/Declaration On February 4, 2020, the Secretary of Health and Human Services determined pursuant to section 564 of the Federal Food, Drug and Cosmetic (FD&C) Act that there is a public health emergency that has a significant potential to affect national security or the health and security of United States (US) citizens living abroad and that involves a novel (new) coronavirus (nCoV) first detected in Wuhan City, Hubei Province, China in 2019 (2019-nCoV). The virus is now named SARS-CoV-2, which causes the illness COVID-19. -

Mark Taylor; [email protected]; (317) 276-5795 (Media) Kevin Hern; Hern Kevin [email protected]; (317) 277-1838 (Investors)
January 29, 2021 Eli Lilly and Company Lilly Corporate Center Indianapolis, Indiana 46285 U.S.A. +1.317.276.2000 www.lilly.com For Release: Immediately Refer to: Mark Taylor; [email protected]; (317) 276-5795 (Media) Kevin Hern; [email protected]; (317) 277-1838 (Investors) Lilly Reports Strong Fourth-Quarter and Full-Year 2020 Financial Results • Revenue in the fourth quarter of 2020 increased 22 percent, driven by volume growth of 24 percent. Excluding bamlanivimab revenue of $871 million, fourth-quarter 2020 revenue grew 7 percent. • Full-year 2020 revenue increased 10 percent, driven by volume growth of 15 percent. Excluding bamlanivimab, full-year 2020 revenue grew 6 percent, driven by volume growth of 11 percent. • Key growth products launched since 2014, consisting of Trulicity, Verzenio, Taltz, Tyvyt, Olumiant, Jardiance, Emgality, Cyramza, Retevmo, Baqsimi and Basaglar contributed nearly 12 percentage points of revenue growth and represented approximately 48 percent of total revenue in the fourth quarter of 2020, or 55 percent of total revenue excluding bamlanivimab. • Fourth-quarter 2020 operating expenses increased 3 percent, driven by higher research and development investments, including expenses of $265 million to develop COVID-19 therapies. • Notable pipeline events included Emergency Use Authorizations from the FDA for both bamlanivimab and baricitinib for the treatment of COVID-19, as well as positive data readouts for donanemab for Alzheimer's disease, tirzepatide for type 2 diabetes and LOXO-305 for cancer. • Fourth-quarter 2020 earnings per share (EPS) increased to $2.32 on a reported basis and $2.75 on a non- GAAP basis. Full year 2020 EPS decreased to $6.79 on a reported basis and increased to $7.93 on a non- GAAP basis. -
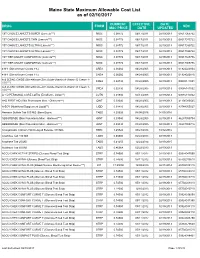
Maine State Maximum Allowable Cost List As of 02/10/2017
Maine State Maximum Allowable Cost List as of 02/10/2017 CURRENT EFFECTIVE DATE DRUG FORM NDC MAC PRICE DATE UPDATED 1ST CHOICE LANCETS SUPER (Lancets***) MISC 0.08772 06/17/2011 02/19/2013 08517065722 1ST CHOICE LANCETS THIN (Lancets***) MISC 0.08772 06/17/2011 02/19/2013 08517015722 1ST CHOICE LANCETS ULTRA (Lancets***) MISC 0.08772 06/17/2011 02/19/2013 08517030722 1ST CHOICE LANCETS ULTRA (Lancets***) MISC 0.08772 06/17/2011 02/19/2013 08517035722 1ST TIER UNILET COMFORTOU (Lancets***) MISC 0.08772 06/17/2011 02/19/2013 08517025736 1ST TIER UNILET COMFORTOU (Lancets***) MISC 0.08772 06/17/2011 02/19/2013 08517065736 4-N-1 (Dimethicone Cream 1%) CREA 0.06060 04/28/2005 02/19/2013 61924020804 4-N-1 (Dimethicone Cream 1%) CREA 0.06060 04/28/2005 02/19/2013 61924020816 A & D ZINC OXIDE (Dimethicone-Zinc Oxide-Vitamin A-Vitamin D Cream 1- CREA 0.02730 04/28/2005 02/19/2013 00085141001 10%***) A & D ZINC OXIDE (Dimethicone-Zinc Oxide-Vitamin A-Vitamin D Cream 1- CREA 0.02730 04/28/2005 02/19/2013 00085141002 10%***) A + D PERSONAL CARE LOTIO (Emollient - Lotion**) LOTN 0.01940 03/13/2008 02/19/2013 00085113002 A+D FIRST AID (Skin Protectants Misc - Ointment***) OINT 0.03040 04/28/2005 02/19/2013 41100080545 A-SOY (Nutritional Supplement Liquid**) LIQD 0.01410 04/28/2005 02/19/2013 83744055027 Abacavir Sulfate Tab 300 MG (Base Equiv) TABS 4.20626 04/08/2016 04/05/2016 ABSORBASE (Skin Protectants Misc - Ointment***) OINT 0.03040 04/28/2005 02/19/2013 46287050704 ABSORBASE (Skin Protectants Misc - Ointment***) OINT 0.03040 04/28/2005 02/19/2013 -

Drug Information Center Highlights of FDA Activities
Drug Information Center Highlights of FDA Activities – 3/1/21 – 3/31/21 FDA Drug Safety Communications & Drug Information Updates: Ivermectin Should Not Be Used to Treat or Prevent COVID‐19: MedWatch Update 3/5/21 The FDA advised consumers against the use of ivermectin for the treatment or prevention of COVID‐19 following reports of patients requiring medical support and hospitalization after self‐medicating. Ivermectin has not been approved for this use and is not an anti‐viral drug. Health professionals are encouraged to report adverse events associated with ivermectin to MedWatch. COVID‐19 EUA FAERS Public Dashboard 3/15/21 The FDA launched an update to the FDA Adverse Event Reporting System (FAERS) Public Dashboard that provides weekly updates of adverse event reports submitted to FAERS for drugs and therapeutic biologics used under an Emergency Use Authorization (EUA) during the COVID‐19 public health emergency. Monoclonal Antibody Products for COVID‐19 – Fact Sheets Updated to Address Variants 3/18/21 The FDA authorized revised fact sheets for health care providers to include susceptibility of SARS‐CoV‐2 variants to each of the monoclonal antibody products available through EUA for the treatment of COVID‐19 (bamlanivimab, bamlanivimab and etesevimab, and casirivimab and imdevimab). Abuse and Misuse of the Nasal Decongestant Propylhexedrine Causes Serious Harm 3/25/21 The FDA warned that abuse and misuse of the nasal decongestant propylhexedrine, sold OTC in nasal decongestant inhalers, has been increasingly associated with cardiovascular and mental health problems. The FDA has recommended product design changes to support safe use, such as modifications to preclude tampering and limits on the content within the device. -

Monoclonal Antibody Playbook
Federal Response to COVID-19: Monoclonal Antibody Clinical Implementation Guide Outpatient administration guide for healthcare providers 2 SEPTEMBER 2021 1 Introduction to COVID-19 Monoclonal Antibody Therapy 2 Overview of Emergency Use Authorizations 3 Site and Patient Logistics Site preparation Patient pathways to monoclonal administration 4 Team Roles and Responsibilities Leadership Administrative Clinical Table of 5 Monoclonal Antibody Indications and Administration Indications Contents Preparation Administration Response to adverse events 6 Supplies and Resources Infrastructure Administrative Patient Intake Administration 7 Examples: Sites of Administration and Staffing Patterns 8 Additional Resources 1 1. Introduction to Monoclonal Therapy 2 As of 08/13/21 Summary of COVID-19 Therapeutics 1 • No Illness . Health, no infections • Exposed Asymptomatic Infected . Scope of this Implementation Guide . Not hospitalized, no limitations . Monoclonal Antibodies for post-exposure prophylaxis (Casirivimab + Imdevimab (RGN)) – EUA Issued. • Early Symptomatic . Scope of this Implementation Guide . Not hospitalized, with limitations . Monoclonal Antibodies for treatment (EUA issued): Bamlanivimab + Etesevimab1 (Lilly) Casirivimab + Imdevimab (RGN) Sotrovimab (GSK/Vir) • Hospital Adminission. Treated with Remdesivir (FDA Approved) or Tocilizumab (EUA Issued) . Hospitalized, no acute medical problems . Hospitalized, not on oxygen . Hospitlaized, on oxygen • ICU Admission . Hospitalized, high flow oxygen, non-invasive ventilation -

“The Silver Sheet” MEDICAL DEVICE QUALITY CONTROL
F-D-C REPORTS — FOUNDED 1939 $665 A YEAR ® www.TheSilverSheet.com“The Silver Sheet” MEDICAL DEVICE QUALITY CONTROL Vol. 13, No. 2 February 2009 THE NEWS THIS ISSUE • RECALLS SOARED TO THEIR HIGHEST POINT EVER IN 2008, with companies recalling a total of 845 devices. Many of the recalls were related to software problems and tainted heparin. “The goal is to try and decrease those recalls,” says Tim Ulatowski, director of CDRH’s Office of Compliance. “So we’re trying to push that number down through effective enforcement actions and industry training.” There is good news, however: The number of Class I recalls fell from 23 in 2007 to only 17 in 2008 – industry’s best Class I showing since 2003. Also, the recall manager for a Virginia hospital chain tells what companies should do to make their recall letters clearer and easier to comply with. “We get some recall letters and we truly don’t know what to do with them,” Bea Haupt says................................................Below • COMPLETE TABLE OF RECALLS FROM 2008 includes 17 Class I (2%), 726 Class II (86%) and 107 Class III (12%) medical device events ............................................................................................................12 • WARNING LETTERS: I-Flow cited for QS and MDR reg violations in relation to its infusion pumps.........66 • IN BRIEF: New GHTF guidance instructs firms on the control of products and services obtained from suppliers; former OSEL Director Larry Kessler is concerned that CDRH doesn’t always follow scientific principles and practices .......................................................................................................Back page Problems Related To Software, Heparin Help Push Recalls To All-Time High Also, FDA And Industry Experts Give Tips For Composing Recall Letters The number of recalled medical devices counting methods are typically negligible; the skyrocketed last year to its highest point ever, agency counted 831 total recalls in FY 2008 and spurred on by a large volume of Class II recalls 664 in FY 2007. -

California Essential Drug List
California Essential Drug List The Essential Drug List (formulary) includes a list of drugs covered by Health Net. The drug list is updated at least monthly and is subject to change. All previous versions are no longer in effect. You can view the most current drug list by going to our website at www.healthnet.com. Refer to Evidence of Coverage or Certificate of Insurance for specific cost share information. For California Individual & Family Plans: Drug Lists Select Health Net Large Group – Formulary (pdf). For Small Business Group: Drug Lists Select Health Net Small Business Group – Formulary (pdf). NOTE: To search the drug list online, open the (pdf) document. Hold down the “Control” (Ctrl) and “F” keys. When the search box appears, type the name of your drug and press the “Enter” key. If you have questions or need more information call us toll free. California Individual & Family Plans (off-Exchange) If you have questions about your pharmacy coverage call Customer Service at 1-800-839-2172 California Individual & Family Plans (on-Exchange) If you have questions about your pharmacy coverage call Customer Service at 1-888-926-4988 Hours of Operation 8:00am – 7:00pm Monday through Friday 8:00am – 5:00pm Saturday Small Business Group If you have questions about your pharmacy coverage call Customer Service at 1-800-361-3366 Hours of Operation 8:00am – 6:00pm Monday through Friday Updated September 1, 2021 Health Net of California, Inc. and Health Net Life Insurance Company are subsidiaries of Health Net, LLC and Centene Corporation. Health Net is a registered service mark of Health Net, LLC Table of Contents What If I Have Questions Regarding My Pharmacy Benefit? ................................... -

SWHPCGV (A6) Scott & White Health Plan Group Value Closed
SWHP Group Value Formulary Federal Employees Health Benefits Program 3rd Quarter 2021 P a g e | 2 Table of Contents What is my prescription drug coverage? ...................................................................... 3 What is the Scott & White Health Plan Group Value Formulary? .................................. 3 How was the formulary created and how are new medications reviewed? .................. 3 Does the formulary ever change? ................................................................................ 3 How am I notified of changes to the formulary? .......................................................... 4 What are brand-name and generic drugs? ................................................................... 4 What is generic substitution? ...................................................................................... 4 What are specialty drugs? ........................................................................................... 4 What are pharmaceutical management procedures?................................................... 5 Are there any restrictions on my coverage? ................................................................. 5 How do I request an exception to the SWHP formulary?.............................................. 5 What drugs are not covered by my prescription drug benefit? ..................................... 5 How much medication does my copayment cover and does my plan cover maintenance medications? ........................................................................................ -

(12) United States Patent (10) Patent No.: US 9,713,596 B2 Hong Et Al
USOO9713596B2 (12) United States Patent (10) Patent No.: US 9,713,596 B2 Hong et al. (45) Date of Patent: Jul. 25, 2017 (54) BAKUCHOL COMPOSITIONS FOR FOREIGN PATENT DOCUMENTS TREATMENT OF POST INFLAMMATORY DE 1900 435 7, 1970 HYPERPGMENTATION DE 3417234 A1 * 11, 1985 JP H1171231 A * 3, 1999 ............... A61K 7.00 (75) Inventors: Mei Feng Hong, Lacey, WA (US); Qi JP 2000-327581 A 11 2000 Jia, Olympia, WA (US); Lidia Alfaro JP 2005325 120 A 11/2005 KR 2000-0007648 A 2, 2000 Brownell, Tacoma, WA (US) WO 2006/122160 A2 11/2006 WO 2008. 140673 A1 11, 2008 (73) Assignee: Unigen, Inc., Lacey, WA (US) (*) Notice: Subject to any disclaimer, the term of this OTHER PUBLICATIONS patent is extended or adjusted under 35 Ohno, O. Watabe, T., Kazuhiko, N., Kawagoshi, M., Uotsu, N., U.S.C. 154(b) by 0 days. Chiba, T., Yamada, M., Yamaguchi, K., Yamada, K., Miyamoto, K., Uemura, D. Inhibitory Effects of Bakuchiol, Bavachin, an (21) Appl. No.: 13/365,172 Isobavachalcone Isolated from Piper loungum on Melainin Produc (22) Filed: Feb. 2, 2012 tion in B 16 Mouse Melanoma Cells. Biosci. Biotechnol. Biochem. 74 (7), 1504-1506 (2010).* Prior Publication Data Hiroyuki Haraguchi, Junji Inoue, Yukiyoshi Tamura and Kenji (65) Mizutani.Antioxidative Components of Psoralea corylifolia US 2012/O2O1769 A1 Aug. 9, 2012 (Leguminosae). Phytother. Res. 16, 539-544 (2002).* Petra Clara Arck, et al. Towards a “free radical theory of graying: melanocyte apoptosis in the aging human hair follicle is an indicator Related U.S. Application Data of oxidative stress induced tissue damage. -

Pharmacy Market Outlook Highlights Keeping You at the Forefront of Drug Price Projections and Market Developments
SUMMER 2021 Pharmacy Market Outlook Highlights Keeping you at the forefront of drug price projections and market developments VIZIENT CENTER FOR PHARMACY PRACTICE EXCELLENCE 1 Pharmacy Market Outlook Summer 2021 Highlights © 2021 Vizient, Inc. All rights reserved. Table of contents Overview Executive summary ......................................................................................................3 Key findings by segment Acute care ......................................................................................................................6 Projections by therapeutic class Specialty pharmaceuticals ..........................................................................................7 Pediatrics .......................................................................................................................8 Oncology ........................................................................................................................9 Infectious disease .......................................................................................................10 Immunomodulators and disease-modifying therapies ........................................11 Plasma products: Intravenous immune globin and albumin ................................12 New: diabetes-related medications .........................................................................13 Management of high-cost therapies Biologics and biosimilars ...........................................................................................14 The evolution -

2021 Bright Formulary (List of Covered Drugs)
2021 Bright Formulary (List of Covered Drugs) Bright Health Individual and Family Plans Colorado PLEASE READ: This document contains information about the drugs Bright Health covers in their Individual and Family plans. This formulary was updated on 09/01/2021. For more recent information or other questions, please contact us at 833-661-1988 or visit www.brighthealthplan.com. PA - Prior Authorization QL - Quantity Limits ST - Step Therapy OTC - Over the i counter Welcome to Bright Enclosed you will find a list of the drugs included in our Bright Health Individual and Family plans from January 1, 2021 - December 31, 2021. As you review, be sure to have your medications on hand so you can confirm your prescriptions are covered and compare dosage and pricing of the drugs you take. Keep in mind, this document includes a comprehensive list of drugs (formulary) included in our Individual and Family plans. For an updated formulary, please contact us. Our contact information, along with the date we last updated the formulary, appears on the front and back cover pages. As a Bright Health member, you must generally use in-network pharmacies to fill your prescriptions. Benefits, formulary, pharmacy network, and/or copayments/coinsurance may change on January 1, 2022, and from time to time during the 2021 calendar year. Sincerely, Your Bright Health team PA - Prior Authorization QL - Quantity Limits ST - Step Therapy OTC - Over the ii counter Frequently Asked Questions: What is a Formulary (drug list)? A formulary is a list of covered drugs selected by Bright Health in consultation with a team of health care providers, which represents the prescription therapies believed to be a necessary part of a quality treatment program. -

Wellcare of South Carolina Medicaid Preferred Drug List
2021 South Carolina Medicaid Comprehensive Preferred Drug List (List of Covered Drugs) Lista integral de medicamentos preferidos de South Carolina Medicaid (Lista de medicamentos cubiertos) WellCare of South Carolina 00 Please read: This document contains information about the drugs we cover in this plan. Please note: The South Carolina Medicaid Preferred Drug List is updated quarterly. Providers, please visit our website at https://www.wellcare.com/South- Carolina/Providers/Medicaid/Pharmacy to view updates to the preferred drug list. Members, please visit our website at https://www.wellcare.com/South-Carolina/Members/Medicaid-Plans/WellCare-of-South-Carolina/Pharmacy- Services to view updates to the preferred drug list. Importante: Este documento contiene información acerca de los medicamentos que tienen cobertura con este plan. Tenga en cuenta lo siguiente: La lista de medicamentos preferidos de South Carolina Medicaid se actualiza cada trimestre. Proveedores: visite nuestro sitio web en https://www.wellcare.com/South- Carolina/Providers/Medicaid/Pharmacy para ver las actualizaciones de la lista de medicamentos preferidos. Miembros: visite nuestro sitio web en https://www.wellcare.com/South-Carolina/Members/Medicaid-Plans/WellCare-of-South-Carolina/Pharmacy- Services para ver las actualizaciones de la lista de medicamentos preferidos. Last updated (4/1/2021) Última actualización (4/1/2021) CAD_67295M State Approved 01282021 ©WellCare 2021 SC1SMDCVR68980M_2021 Drug Name Preference Details Coverage Details *Adhd/Anti-Narcolepsy/Anti-