Micrus Pro User Guide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cennik Urządzeń W Ofercie Red Bull MOBILE Siema
Cennik urządzeń w Ofercie Red Bull MOBILE SieMa Obowiązuje od dnia 18.04.2019 do wyczerpania stanów magazynowych objętych niniejszym Cennikiem lub jego odwołania. Red Bull MOBILE Model SieMa Smartfona SieMa Smartfona SieMa Smartfona SieMa Wypas 49 69 89 199 Alcatel 1 DS 6 6 6 6 Alcatel 1s 36 6 6 6 Alcatel 1X DS 6 6 6 6 Alcatel 30.25 6 6 6 6 BlackBerry Key2 2046 1916 1446 Google Chromecast 2 2015 6 6 6 6 Hammer Energy 18x9 DS 366 236 6 6 HTC Desire 12s 226 96 6 6 HTC U12+ DS 1546 1426 996 6 Huawei Mate 10 Lite DS 396 276 6 6 Huawei Mate 10 Lite DS + Mate 10 Lite DS 1316 1016 696 6 Huawei Mate 10 Pro DS 1506 1386 966 6 Huawei Mate 20 Lite DS 726 596 176 6 Huawei Mate 20 Lite DS + Etui Mate 20 Lite DS 756 626 196 6 Huawei Mate 20 Pro DS 3066 2936 2526 Huawei MediaPad T3 10 LTE 236 116 6 6 Huawei P smart 2019 396 276 6 6 Huawei P smart DS 86 6 6 6 Huawei P20 64GB + Band 2 Pro 1036 896 496 6 Huawei P20 64GB DS 1036 896 496 6 Huawei P20 DS 1216 1096 676 6 Huawei P20 Lite DS 556 426 16 6 Huawei P20 Lite DS + Etui P20 Lite 586 456 36 6 Huawei P20 Lite DS + MediaPad T3 7" WIFI 626 466 6 6 Huawei P20 Pro DS 1416 1286 876 6 Huawei P30 2386 2266 1846 Huawei P30 Pro 2946 2816 2396 Huawei Y5 2018 DS 6 6 6 6 Huawei Y6 2018 DS 76 6 6 6 Huawei Y6 2018 DS + Y6 2018 DS 386 236 6 6 Huawei Y6 2019 196 66 6 6 iPhone 6S 32GB 1236 1096 696 6 P4 SP. -

Why Can't I Use Bluetooth to Transfer Files to Another Device? Applicable Products: Problem: 1
Why can't I use Bluetooth to transfer files to another device? Applicable products: Problem: 1. The user is unable to send files using Bluetooth. 2. The user is unable to receive files using Bluetooth. Solution: 1. The distance between your phone and the other Bluetooth device may have exceeded the maximum operational range of Bluetooth devices (10 meters). In this case, move your phone closer to the Bluetooth device, and try again. 2. The other Bluetooth device may not have responded to your file transfer request in time. You can try sending the request again and then remind the other party to confirm the transfer more quickly. 3. Your phone is not paired with the Bluetooth device. In this case, first make sure that Bluetooth is enabled on both devices, and that both devices are visible to nearby Bluetooth devices (for laptops, check that the file receiving screen is displayed). Then send the file. 4. Pair the two devices first, and then send the file. 5. Check whether the other Bluetooth device supports the file format you are trying to send. 6. If the other Bluetooth device supports the given file format, try sending the file again. Note: 1. iPhones can be paired with Huawei phones using Bluetooth, but do not support file transfer. 2. The music app preinstalled in your Huawei phone does not support Bluetooth sharing. Therefore, you cannot share music downloaded within the app with other Bluetooth devices. Open the Files app, touch Local > Internal storage > Music, find the name of the song you want to share, and touch to share it with other Bluetooth devices. -

HR Kompatibilitätsübersicht
HR-imotion Kompatibilität/Compatibility 2018 / 11 Gerätetyp Telefon 22410001 23010201 22110001 23010001 23010101 22010401 22010501 22010301 22010201 22110101 22010701 22011101 22010101 22210101 22210001 23510101 23010501 23010601 23010701 23510320 22610001 23510420 Smartphone Acer Liquid Zest Plus Smartphone AEG Voxtel M250 Smartphone Alcatel 1X Smartphone Alcatel 3 Smartphone Alcatel 3C Smartphone Alcatel 3V Smartphone Alcatel 3X Smartphone Alcatel 5 Smartphone Alcatel 5v Smartphone Alcatel 7 Smartphone Alcatel A3 Smartphone Alcatel A3 XL Smartphone Alcatel A5 LED Smartphone Alcatel Idol 4S Smartphone Alcatel U5 Smartphone Allview P8 Pro Smartphone Allview Soul X5 Pro Smartphone Allview V3 Viper Smartphone Allview X3 Soul Smartphone Allview X5 Soul Smartphone Apple iPhone Smartphone Apple iPhone 3G / 3GS Smartphone Apple iPhone 4 / 4S Smartphone Apple iPhone 5 / 5S Smartphone Apple iPhone 5C Smartphone Apple iPhone 6 / 6S Smartphone Apple iPhone 6 Plus / 6S Plus Smartphone Apple iPhone 7 Smartphone Apple iPhone 7 Plus Smartphone Apple iPhone 8 Smartphone Apple iPhone 8 Plus Smartphone Apple iPhone SE Smartphone Apple iPhone X Smartphone Apple iPhone XR Smartphone Apple iPhone Xs Smartphone Apple iPhone Xs Max Smartphone Archos 50 Saphir Smartphone Archos Diamond 2 Plus Smartphone Archos Saphir 50x Smartphone Asus ROG Phone Smartphone Asus ZenFone 3 Smartphone Asus ZenFone 3 Deluxe Smartphone Asus ZenFone 3 Zoom Smartphone Asus Zenfone 5 Lite ZC600KL Smartphone Asus Zenfone 5 ZE620KL Smartphone Asus Zenfone 5z ZS620KL Smartphone Asus -

Device Compatibility
Device compatibility Check if your smartphone is compatible with your Rexton devices Direct streaming to hearing aids via Bluetooth Apple devices: Rexton Mfi (made for iPhone, iPad or iPod touch) hearing aids connect directly to your iPhone, iPad or iPod so you can stream your phone calls and music directly into your hearing aids. Android devices: With Rexton BiCore devices, you can now also stream directly to Android devices via the ASHA (Audio Streaming for Hearing Aids) standard. ASHA-supported devices: • Samsung Galaxy S21 • Samsung Galaxy S21 5G (SM-G991U)(US) • Samsung Galaxy S21 (US) • Samsung Galaxy S21+ 5G (SM-G996U)(US) • Samsung Galaxy S21 Ultra 5G (SM-G998U)(US) • Samsung Galaxy S21 5G (SM-G991B) • Samsung Galaxy S21+ 5G (SM-G996B) • Samsung Galaxy S21 Ultra 5G (SM-G998B) • Samsung Galaxy Note 20 Ultra (SM-G) • Samsung Galaxy Note 20 Ultra (SM-G)(US) • Samsung Galaxy S20+ (SM-G) • Samsung Galaxy S20+ (SM-G) (US) • Samsung Galaxy S20 5G (SM-G981B) • Samsung Galaxy S20 5G (SM-G981U1) (US) • Samsung Galaxy S20 Ultra 5G (SM-G988B) • Samsung Galaxy S20 Ultra 5G (SM-G988U)(US) • Samsung Galaxy S20 (SM-G980F) • Samsung Galaxy S20 (SM-G) (US) • Samsung Galaxy Note20 5G (SM-N981U1) (US) • Samsung Galaxy Note 10+ (SM-N975F) • Samsung Galaxy Note 10+ (SM-N975U1)(US) • Samsung Galaxy Note 10 (SM-N970F) • Samsung Galaxy Note 10 (SM-N970U)(US) • Samsung Galaxy Note 10 Lite (SM-N770F/DS) • Samsung Galaxy S10 Lite (SM-G770F/DS) • Samsung Galaxy S10 (SM-G973F) • Samsung Galaxy S10 (SM-G973U1) (US) • Samsung Galaxy S10+ (SM-G975F) • Samsung -
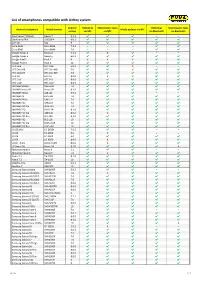
List of Smartphones Compatible with Airkey System
List of smartphones compatible with AirKey system Android Unlocking Maintenance tasks Unlocking Maintenance tasks Android smartphone Model number Media updates via NFC version via NFC via NFC via Bluetooth via Bluetooth Asus Nexus 7 (Tablet) Nexus 7 5.1.1 ✔ ✔ ✔ – – Blackberry PRIV STV100-4 6.0.1 ✔ ✔ ✔ ✔ ✔ CAT S61 S61 9 ✔ ✔ ✔ ✔ ✔ Doro 8035 Doro 8035 7.1.2 – – – ✔ ✔ Doro 8040 Doro 8040 7.0 – – – ✔ ✔ Google Nexus 4 Nexus 4 5.1.1 ✔ X ✔ – – Google Nexus 5 Nexus 5 6.0.1 ✔ ✔ ✔ ✔ ✔ Google Pixel 2 Pixel 2 9 X X X ✔ ✔ Google Pixel 4 Pixel 4 10 ✔ ✔ ✔ ✔ ✔ HTC One HTC One 5.0.2 ✔ ✔ X – – HTC One M8 HTC One M8 6.0 ✔ ✔ X ✔ ✔ HTC One M9 HTC One M9 7.0 ✔ ✔ ✔ ✔ ✔ HTC 10 HTC 10 8.0.0 ✔ X X ✔ ✔ HTC U11 HTC U11 8.0.0 ✔ ✔ ✔ ✔ ✔ HTC U12+ HTC U12+ 8.0.0 ✔ ✔ ✔ ✔ ✔ HUAWEI Mate 9 MHA-L09 7.0 ✔ ✔ ✔ ✔ ✔ HUAWEI Nexus 6P Nexus 6P 8.1.0 ✔ ✔ ✔ ✔ ✔ HUAWEI P8 lite ALE-L21 5.0.1 ✔ ✔ ✔ – – HUAWEI P9 EVA-L09 7.0 ✔ ✔ ✔ ✔ ✔ HUAWEI P9 lite VNS-L21 7.0 ✔ ✔ ✔ ✔ ✔ HUAWEI P10 VTR-L09 7.0 ✔ ✔ ✔ ✔ ✔ HUAWEI P10 lite WAS-LX1 7.0 ✔ ✔ ✔ ✔ ✔ HUAWEI P20 EML-L29 8.1.0 ✔ ✔ ✔ ✔ ✔ HUAWEI P20 lite ANE-LX1 8.0.0 ✔ X ✔ ✔ ✔ HUAWEI P20 Pro CLT-L29 8.1.0 ✔ ✔ ✔ ✔ ✔ HUAWEI P30 ELE-L29 10 ✔ ✔ ✔ ✔ ✔ HUAWEI P30 lite MAR-LX1A 10 ✔ ✔ ✔ ✔ ✔ HUAWEI P30 Pro VOG-L29 10 ✔ ✔ ✔ ✔ ✔ LG G2 Mini LG-D620r 5.0.2 ✔ ✔ ✔ – – LG G3 LG-D855 5.0 ✔ X ✔ – – LG G4 LG-H815 6.0 ✔ ✔ ✔ ✔ ✔ LG G6 LG-H870 8.0.0 ✔ X ✔ ✔ ✔ LG G7 ThinQ LM-G710EM 8.0.0 ✔ X ✔ ✔ ✔ LG Nexus 5X Nexus 5X 8.1.0 ✔ ✔ ✔ ✔ X Motorola Moto X Moto X 5.1 ✔ ✔ ✔ – – Motorola Nexus 6 Nexus 6 7.0 ✔ X ✔ ✔ ✔ Nokia 7.1 TA-1095 8.1.0 ✔ ✔ X ✔ ✔ Nokia 7.2 TA-1196 10 ✔ ✔ ✔ -

Cennik Urządzeń Dla Ofert T-Mobile – Bez Limitu Oraz Ofert T
Cennik urządzeń dla ofert T-Mobile – Bez limitu oraz Ofert T. 1. W okresie od dnia 14.06.2019 r. do wyczerpania zapasów właściwych dla ofert promocyjnych lub ich wycofania T-Mobile Polska S.A. („Operator”) oferuje sprzęt zgodnie z poniższym. Wymienione poniżej urządzenia i warunki cenowe dotyczą Warunków Ofert Promocyjnych odsyłających w swej treści do niniejszego dokumentu. 2. Abonament podany jest poniżej wyłącznie w celu dodatkowego ułatwienia w znalezieniu właściwej kolumny. W przypadku ofert dla abonentów przenoszących numer z sieci innego operatora podany Abonament jest właściwy dla większości Cykli Rozliczeniowych czasu określonego Umowy i uwzględnia ewentualny upust za zgody marketingowe. Wartości podane poniżej w tabelach w ppkt. 2.2. oraz w 2.4 (za wyjątkiem wierszy tabel zatytułowanych „Typowa suma Abonamentu i raty”) wskazują wyrażoną w złotych polskich wysokość pierwszej raty wynikajacej z Umowy Sprzedaży na Raty. „Typowa suma Abonamentu i raty” to suma Abonamentu i miesięcznej stałej raty (tj. innej niż pierwsza), właściwa dla większości Cykli Rozliczeniowych czasu określonego Umowy. Uwzględnia ona ewentualny upust za zgody marketingowe. Kwota „Typowej sumy Abonamentu i raty” podawana jest poniżej wyłącznie w celu dodatkowego ułatwienia w znalezieniu właściwej kolumny. Ceny sprzętu sprzedawanego na raty podane są w pkt 3 poniżej. Telefony dostępne w Warunkach Oferty Promocyjnej „T1 2GB i T2 5GB z telefonem na 24 miesiące w T-Mobile”, Warunkach Oferty Promocyjnej „T-Mobile - Bez Limitu z telefonem na 24 miesiące w T-Mobile. -

Smartphone Camera Modules Comparison 2020 Vol 3: Focus on Huawei
REVERSE COSTING® – STRUCTURAL, PROCESS & COST REPORT P9 P10 P20 Pro Mate 20 Pro P30 Pro Mate 30 Pro P40 Pro Smartphone Camera Modules Comparison 2020 Vol. 3 Focus on Huawei SP20525 - IMAGING report by Audrey LAHRACH Physical Analysis by Véronique LE TROADEC & Guillaume CHEVALIER December 2020 – Sample 22 bd Benoni Goullin 44200 NANTES - FRANCE +33 2 40 18 09 16 [email protected] www.systemplus.fr ©2020 by System Plus Consulting | SP20525 – Smartphone Camera Module Comparison 2020 Vol 3: Focus on Huawei | Sample 1 Table of Contents Cost Comparison 96 Overview / Introduction 5 o CIS Die Cost Comparison o Executive Summary o Reverse Costing Methodology o Front Facing o Glossary o Rear Facing Company Profile & Supply Chain 9 o Total CIS Die Price o Huawei, Sony, Omnivision o CM Cost Comparison o Players & Market Forecast Physical Comparison 19 o Front Facing o Camera Module Overview o Rear Facing o Summary of the Physical Analysis o Total CM Price o Huawei P40 Pro Detailed Physical Analysis 122 o Huawei P30 Pro ➢ P Series o Huawei P20 Pro o P40 Pro ✓ Front & Rear Camera o Huawei P10 o P30 Pro o Huawei P9 ✓ Front & Rear Camera o Mate 30 Pro o P20 Pro o Mate 20 Pro ✓ Front & Rear Camera Physical Evolution 55 o P10 o Display Evolution ✓ Front & Rear Camera o Huawei Front Camera Evolution P Series o P9 o Huawei Rear Camera Evolution P Series ✓ Front & Rear Camera o Huawei Front Camera Evolution/Comparison Mate vs P Series ➢ Mate Series o Huawei Rear Camera Evolution/Comparison Mate vs P Series o Mate 30 Pro o Camera Module Description ✓ Front & Rear Camera o Bonding & Stacking evolution (Sony & Omnivision) o Mate 20 Pro ✓ Front & Rear Camera Feedbacks 240 SystemPlus Consulting services 242 ©2020 by System Plus Consulting | SP20525 – Smartphone Camera Module Comparison 2020 Vol 3: Focus on Huawei | Sample 2 Executive Summary Overview / Introduction o Executive Summary o Reverse Costing Huawei is among the top 3 smartphone OEMs today. -

Mobile- H2 2018
Barometer of mobile Internet connections in Turkey Publication of August 12 th , 2019 H2 2018 – H1 2019 nPerf is a trademark owned by nPerf SAS, 87 rue de Sèze 69006 LYON – France. Contents 1 Summary of results ...................................................................................................................... 2 1.1 Summary table and nPerf score, all technologies combined .............................................. 2 1.2 Our analysis ........................................................................................................................... 3 2 Overall results 2G/3G/4G ............................................................................................................. 3 2.1 Data amount and distribution ............................................................................................... 3 2.2 Success rate 2G/3G/4G ........................................................................................................ 4 2.3 Download speed 2G/3G/4G .................................................................................................. 4 2.4 Upload speed 2G/3G/4G ....................................................................................................... 6 2.5 Latency 2G/3G/4G ................................................................................................................ 7 2.6 Browsing test 2G/3G/4G....................................................................................................... 8 2.7 Streaming test 2G/3G/4G .................................................................................................... -

Cennik Telefonów W Ofercie SIM STANDARD Dla Firm
dla Firm Cennik telefonów w Ofercie SIM STANDARD dla Firm Obowiązuje od dnia 21.12.2018 do wyczerpania stanów magazynowych objętych niniejszym Cennikiem lub jego odwołania. SIM STANDARD dla Firm Model + 0 + 10 + 20 + 30 + 40 + 50 + 60 +80 + 100 + 120 + 140 +160 + 180 Alcatel 1 DS 199 1 1 1 1 1 1 1 1 1 1 1 1 Alcatel 1X DS 169 1 1 1 1 1 1 1 1 1 1 1 1 Alcatel 30.25 129 1 1 1 1 1 1 1 1 1 1 1 1 BlackBerry Key2 2049 1619 1419 1239 1059 879 499 99 Google Chromecast 2 2015 159 1 1 1 1 1 1 1 1 1 1 1 1 HTC Desire 12 DS 299 79 1 1 1 1 1 1 1 1 1 1 1 HTC Desire 12+ DS 479 259 49 1 1 1 1 1 1 1 1 1 1 HTC Desire 12+ DS + Desire 12+ DS 949 519 319 139 1 1 1 1 1 1 1 1 HTC U12+ DS 2149 1719 1519 1339 1159 979 599 199 Huawei Mate 10 Lite DS 729 299 99 1 1 1 1 1 1 1 1 1 Huawei Mate 10 Pro DS 2019 1599 1399 1219 1039 859 479 79 Huawei Mate 20 Lite DS 969 539 339 159 1 1 1 1 1 1 1 1 Huawei Mate 20 Lite DS + Etui Mate 20 999 569 369 189 1 1 1 1 1 1 1 1 Lite DS Huawei Mate 20 Pro DS 3169 2739 2529 2349 2169 1989 1619 1219 889 499 119 Huawei MediaPad T3 10 LTE 489 279 59 1 1 1 1 1 1 1 1 1 1 Huawei P smart DS 559 339 129 1 1 1 1 1 1 1 1 1 1 Huawei P smart DS + Huawei P Smart 579 359 149 1 1 1 1 1 1 1 1 1 1 Flip Cover Huawei P20 64GB DS 1489 1059 859 679 499 319 1 1 1 1 1 1 P4 SP. -
Størrelsesskema for Universal Smartphone Holdere
Størrelsesskema for universal smartphone holdere iPhone 6 INCH 6.5 INCH > 6.5 INCH Apple iPhone 11 Pro 5.8" Apple iPhone 11 Pro Max 6.5" iPhone XS 5.8" Apple iPhone XS Max 6.5" iPhone X 5.8" Apple iPhone XR 6.1" iPhone 8 Plus 5.5" Apple iPhone 11 6.1" iPhone 8 4.7" iPhone 7 Plus 5.5" iPhone 7 4.7" iPhone SE 4.0" iPhone 6s Plus 5.5" iPhone 6s 4.7" iPhone 6 Plus 5.5" iPhone 6 4.7" iPhone 5s 4.0" iPhone 5c 4.0" iPhone 5 4.0" iPhone 4s 3.5" Samsung 6 INCH 6.5 INCH > 6.5 INCH Samsung Galaxy Xcover 4s 5.0" Samsung Galaxy A20s 6.5" Samsung Galaxy A70s 6.7" Samsung Galaxy A2 Core 5.0" Samsung Galaxy M30s 6.4" Samsung Galaxy Fold 5G 7.3" Samsung Galaxy S10e 5.8" Samsung Galaxy M10s 6.4" Samsung Galaxy Fold 7.3" Samsung Galaxy A40 5.9" Samsung Galaxy A30s 6.4" Samsung Galaxy A90 5G 6.7" Samsung Galaxy A20e 5.8" Samsung Galaxy A50s 6.4" Samsung Galaxy Note10+ 5G 6.8" Samsung Galaxy J2 Core 5.0" Samsung Galaxy Note10 5G 6.3" Samsung Galaxy Note10+ 6.8" Samsung Galaxy On6 5.6" Samsung Galaxy Note10 6.3" Samsung Galaxy S10 5G 6.7" Samsung Galaxy A10s 6.2" Samsung Galaxy A80 6.7" Samsung Galaxy A10e 5.83" Samsung Galaxy A70 6.7" Samsung Galaxy S10+ 6.4" Samsung Galaxy S10 6.1" Samsung Galaxy M40 6.3" Samsung Galaxy M30 6.4" Samsung Galaxy M20 6.3" Samsung Galaxy M10 6.22" Samsung Galaxy A60 6.3" Samsung Galaxy A50 6.4" Samsung Galaxy A30 6.4" Samsung Galaxy A20 6.4" Samsung Galaxy A10 6.2" Samsung Galaxy A8s 6.4" Samsung Galaxy A6s 6.0" Samsung Galaxy A9 6.3" Samsung Galaxy A7 6.0" Samsung Galaxy Note9 6.4" Samsung Galaxy J6+ 6.0" Samsung Galaxy J4 -

List of Supported Devices
List of Compatible Devices Spike By: ikeGPS For: Spike Customers V 1.0.0.0 Date: 2020 1 Table of Contents Table of Contents Supported Android Smartphone and Tablet Devices Supported Apple iOS Smartphone and Tablet Devices 2 Introduction The following is a list of devices that are compatible with Spike. To us, compatible means more than just, it works. Compatible means that it has been tested and adheres to the accuracy standards that we hold ourselves to at ikeGPS. If you have any questions at all, please feel free to reach out to [email protected]. Supported Android Smartphone and Tablet Devices Device Photo Point-to- Target Comment Measure Point Location Asus ZenPad 3S 10 ✓ ✗ ✗ Asus Zenpad Z8 ✓ ✗ ✗ Supported for Photo Measure but not recommended for P2P or Target Location Asus Zenpad Z10 ✓ ✓ ✓ HTC One M8 ✓ ✗ ✗ HTC One Mini ✓ ✗ ✗ HTC U11 ✓ ✗ ✗ iNew L1 ✓ ✗ ✗ Kyocera Dura Force PRO ✓ ✓ ✓ 2 LGV20 ✓ ✗ ✗ Motorola Moto G ✓ ✗ ✗ Supported for Photo Measure but not recommended Motorola Moto X XT1052 ✓ ✗ ✗ Supported for Photo Measure but not recommended Motorola Moto X XT1053 ✓ ✗ ✗ Supported for Photo Measure but not recommended Nexus 5 ✓ ✓ ✓ 3 Nexus 5X ✓ ✓ ✓ Nexus 6 ✓ ✓* ✓ * Supported for Point-to-Point, but cannot guarantee +/-3% accuracy Nexus 6P ✓ ✓* ✓ * Supported for Point-to-Point, but cannot guarantee +/-3% accuracy Nexus 7 ✓ ✓* ✓ * Supported for Point-to-Point, but cannot guarantee +/-3% accuracy Samsung Galaxy A20 ✓ ✓ ✓ Samsung Galaxy J7 ✓ ✗ ✓ Prime Samsung GALAXY Note3 ✓ ✓* ✓ * Supported for Point-to-Point, but cannot guarantee +/-3% -

Huawei Mate 10 Pro Launch
Huawei Mate 10 Pro Launch Huawei Mate 10 Pro Launch 1 / 4 2 / 4 The Mate 10 Pro was a defining handset for Huawei when it launched in 2017. Every bit as capable as its Samsung, LG and Apple competition, .... Huawei Mate 10 Pro price and release date. The Huawei Mate 10 Pro launch price was £699 ($799, AU$1,099) which saw it slide it below some ... 1. huawei mate launch 2. huawei mate launch 2019 3. huawei mate 30 pro launch Huawei Mate 10 Pro smartphone was launched in October 2017. The phone comes with a 6.00-inch touchscreen display with a resolution of 1080x2160 pixels ... huawei mate launch huawei mate launch, huawei mate launch date, huawei mate launch 2019, huawei mate 30 pro launch date, huawei mate 30 pro launch, huawei mate 30 launch, huawei mate 30 pro launch date in india, huawei mate 30 launch date, huawei mate x launch date, huawei mate 40 pro launch date, huawei launch mate 30, huawei launch mate 40, huawei mate 40 launch date, huawei mate x launch, huawei mate 40 launch event dominasi slumdog millionaire Update: Since the launch of the Huawei P20 and P20 Pro, the Chinese firm's previous flagships have been dropped in price. The Huawei Mate 10 Pro launched .... Pricing and availability. Only the Mate 10 Pro is available in the U.S., and it was originally going to launch on AT&T — a first for Huawei. The deal .... Check full specification of Huawei Mate 10 Pro Mobiles with its features, reviews & comparison at Gadgets ..