ERYTECH Provides Business Update and Financial Results for the First Half
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ANNUAL FINANCIAL REPOR T / APRIL R Egistration D Ocument / Y
ANNUAL FINANCIAL REPORT / APRIL Registration Document / Year 2013 REGISTRATION DOCUMENT REGISTRATION financial annual Report 2013 ANNUAL FINANCIAL REPORT 2013 REGISTRATION DOCUMENT Pursuant to Article 212-13 of the general regulations of the French securities regulator (Autorité des Marchés Financiers, AMF), the present annual financial report was filed (in French) with the AMF as a registration document on March 31st, 2014. It may be used in market transactions provided that it is accompanied by a copy of a prospectus that has been duly approved by the AMF. This document has been drawn up by the issuer and is the responsibility of its signatory. Financial information manager: Marc Le Doze Chief Financial Officer APRIL, Tel. +33 (0)4 72 36 18 85 CONTENTS PART 1 PART 4 MAIN DOCUMENT STATUTORY FINANCIAL STATEMENTS 06 Responsibility for this financial report and audits 168 APRIL statutory financial statements and notes at 08 General information on the company and its share capital December 31st, 2013 16 Information on the company’s activities 200 Statutory Auditors’ general report on the annual financial statements 33 Net worth – Financial position – Earnings 201 Special Statutory Auditors’ report on regulated agreements 38 Corporate Governance and commitments 46 Recent developments and outlook 203 General Shareholders’ Meeting Resolutions PART 2 PART 5 MANAGEMENT REPORT APPENDIX 48 Management report 216 Description of the share buyback program 80 Five-year financial summary 217 Index of headings 81 Chairman of the Board of Directors’ report -

Myhealth International Health Insurance at Your Doorstep
MyHEALTH International health insurance at your doorstep Medical Expenses covered from the 1st dollar spent Download our Easy Claim mobile app for quicker claims reimbursement! april-international.com Please print only if necessary HEALTH INSURANCE MADE EASIER MyHEALTH is designed for global citizens seeking long term health coverage at home and abroad. This policy provides comprehensive and fully flexible cover for medical expenses plus assistance for you and your family. 2020 Global Banking and Finance Awards Best Individual Health Insurance Company Asia Pacific 2020 Best Health Insurance Product Asia Pacific 2020 Service Initiative of the Year 2020 APRIL International is part of the APRIL group which was founded in France more than 30 years ago. The APRIL group has grown steadily to the point where we now look after close to 6 million policyholders worldwide who, at the last count, represent some 86 different nationalities and are located in more than 120 countries around the globe. MyHEALTH is underwritten by the Post & Telecommunication Joint Stock Insurance Corporation (PTI), a Vietnam-based company active in the non-life insurance industry. The Company offers over 100 non-life and health insurance products to individuals and corporate clients. These products are categorized into four segments: property and engineering insurance, motor vehicle insurance, marine insurance and health and accident insurance. The strong partnership of APRIL International and PTI ensures that you receive the benefits of our combined international and regional experience; and financial strength. Together we place trust at the centre of our relationships and rely on three core principles 1 2 3 TRANSPARENCY RELIABILITY SUSTAINABILITY Easy to understand Always there when Getting the best & straightforward you need us deal for you 02 PLAN STRUCTURE My HEALTH is extremely flexible, allowing you to mix and match modules to design your ideal health insurance plan. -

Crystal Studies Benefits 2021
STUDIES EASY CLAIM HOLIDAYS INTERNSHIPS TO SUBMIT YOUR CLAIMS FOR AU PAIR REIMBURSEMENT PLACEMENTS IN JUST A FEW COVER fOR UP TO CLICKS 12 MONTHS CRYSTAL STUDIES A COMpREhENSIvE INSURANCE pACKAgE FOR STUDYINg ABROAD 2021 COVI D-19 covered Download our free mobile app Easy Claim! CRYSTAL STUDIES, COMpREhENSIvE INSURANCE FOR STUDYINg ABROAD! Crystal Studies is an insurance solution designed for students and schoolchildren living abroad for up to a year for the purposes of travel, language training, au pair placements, internships or studies. You will be studying abroad for more than 12 months? Check out Expat Student , our insurance solution especially designed for studies of 12 months and more worldwide! WHY TAkE OUT INTERNATIONAL INSURANCE? Healthcare costs abroad vary by country and can be extremely high. Quite frequently, your home country healthcare benefits arent's valid abroad. You won't be able to rely on Social Security cover: > cover isn't granted in all cases, > reimbursements are based on rates. Healthcare costs abroad can be for higher than those in your home 5 country. In Europe, the European Health Insurance Card (EHIC) provides insufficient cover: > limited access to healthcare professionals and care facilities within the public system (with sometimes very long waiting times), > no free choice of doctor, > no certainty that your card will be accepted, 5 > your own contribution to costs may sometimes be significant. Bank card cover is only valid for trips of 3 months maximum: With a “basic” bank card, there's a minimum excess of €50 per item and total reimbursement is capped at €11,000. -
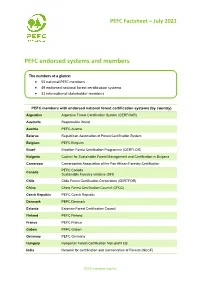
PEFC Endorsed Systems and Members
PEFC Factsheet – July 2021 PEFC endorsed systems and members The numbers at a glance: • 55 national PEFC members • 49 endorsed national forest certification systems • 31 international stakeholder members PEFC members with endorsed national forest certification systems (by country) Argentina Argentine Forest Certification System (CERFOAR) Australia Responsible Wood Austria PEFC Austria Belarus Republican Association of Forest Certification System Belgium PEFC Belgium Brazil Brazilian Forest Certification Programme (CERFLOR) Bulgaria Council for Sustainable Forest Management and Certification in Bulgaria Cameroon Cameroonian Association of the Pan African Forestry Certification PEFC Canada Canada Sustainable Forestry Initiative (SFI) Chile Chile Forest Certification Corporation (CERTFOR) China China Forest Certification Council (CFCC) Czech Republic PEFC Czech Republic Denmark PEFC Denmark Estonia Estonian Forest Certification Council Finland PEFC Finland France PEFC France Gabon PAFC Gabon Germany PEFC Germany Hungary Hungarian Forest Certification Non-profit Ltd. India Network for Certification and Conservation of Forests (NCCF) PEFC membership list Indonesia Indonesian Forestry Certification Cooperation (IFCC) Ireland PEFC Ireland Italy PEFC Italy Japan Sustainable Green Ecosystem Council (SGEC) Latvia PEFC Latvia Luxembourg PEFC Luxembourg Malaysia Malaysian Timber Certification Council (MTCC) Netherlands PEFC Netherlands New Zealand New Zealand Forest Certification Association (NZFCA) North Macedonia The Council for Sustainable Forest -

He Reference Document
The Reference document 2007 WorldReginfo - 96cdd1b6-8edd-4a6d-b9d6-e2d64bce6bce Document de référence 2007 Pursuant to Article 212-13 of the general Financial information managers: regulations of the French securities regulator Dominique Godet Chief Financial Officer, APRIL GROUP, (Autorité des Marchés Financiers, AMF), the Tel: +33 (0)4 72 36 18 98 present reference document was filed in French Jean-Marc Atlan with the AMF as an annual report on March 14th, Kaelia 2008 (filing ref : D.08-01 11). Tel: +33 (0)4 72 00 35 81 It may be used in market transactions provided that it is accompanied by a copy of a prospectus that has been duly approved by the AMF. WorldReginfo - 96cdd1b6-8edd-4a6d-b9d6-e2d64bce6bce Contents 01 Main Document 04 Statutory financial statements Responsibility for this reference document and audits .................................................... p.4 APRIL GROUP statutory financial statements at Dec 31st, 2007 .............................p.151 General information on the company and its share capital............................................ p.6 Highlights ............................................................................................................................................p.154 Information on the company’s activities ............................................................................... p.15 Notes to the statutory financial statements of APRIL GROUP SA Net worth-financial position-earnings ................................................................................... p.32 for -

Lyon, European City of the Future ? 2019 FOREWORD
Why invest in Lyon, European city of the future ? 2019 FOREWORD A historic year for Lyon’s commercial real estate markets In 2018, the combined take-up of light industrial premises and logistics warehouses in the Lyon market exceeded 820,000 m² and confirmed Lyon as one of the most dynamic industrial markets in Europe. The office take-up in 2018 also hit the historic market record of 331 700 m². The volume has never been so high, and the number of transactions exceeded the 600 mark for the first time. Investment volumes are also impressive with more than Laurent Vallas 1.5 billion euros invested in the Lyon commercial real estate market in 2018. JLL Head of JLL Lyon Lyon is also one of the most dynamic European cities in terms of business and leisure tourism. Voted “Europe’s leading City Break Destination” in 2016, Lyon has been incluted in the New York Times’ prestigious annual international selection of “52 Places to Go” in 2019. It is not a surprise that the hotel market has never been so flourishing with more than five million hotel nights sold in 2018. Finally, Lyon remains one of the most sought-after cities in France for the retail market, a trend that has been enhanced by the new openings in the Grolée-Carnot sector and the spectacular conversion of the Grand Hôtel-Dieu (16 000 m² of retail and restaurant space), a vibrant venue for a new kind of retailing with a resolute focus on the growing interest in “shopping as an experience”. -

2020 Insurance M&A Outlook
2020 insurance M&A outlook Pursuing growth amid uncertainty 2020Brochure insurance / report M&A title outlook goes here | Pursuing | Section growth title goes amid here uncertainty Contents Overview and outlook 1 2019 in review 1 2020 outlook 7 2020 insurance M&A drivers and trends 8 Portfolio optimization 8 Improving the customer experience 8 Maturing InsurTech market 9 Integration imperative 10 Accelerating insurance innovation 11 Accounting, regulatory, and tax influences on M&A activity 11 Moving forward on 2020 insurance M&A opportunities 14 Appendix 15 Spotlight: Insurance industry M&A in major global markets 15 2 2020 insurance M&A outlook | Pursuing growth amid uncertainty Overview and outlook Projected economic, interest rate, and financial market uncertainty— However, that proved not to be the case in the property and casualty along with a presidential election—are among the headwinds that (P&C) sector, as evidenced by declines in deal volume, aggregate may give pause to insurance companies contemplating M&A in 2020. deal value, and average deal value (figure 1). In the life and health Despite these potential challenges, companies continue to view (L&H) sector, the decrease in the aggregate deal volume was not alliances, investments, and acquisitions as attractive options when material, and the average deal value actually increased. However, market factors make organic growth more difficult. Organizations these metrics were heavily influenced by the announced $6.3 billion that select targets that are accretive, synergistic, and -

Commission for Polish Relief Records, 1939-1949
http://oac.cdlib.org/findaid/ark:/13030/tf4b69n6pg No online items Register of the Commission for Polish Relief Records, 1939-1949 Processed by Natalia A. Porfirenko; machine-readable finding aid created by James Ryan Hoover Institution Archives Stanford University Stanford, California 94305-6010 Phone: (650) 723-3563 Fax: (650) 725-3445 Email: [email protected] © 1997 Hoover Institution Archives. All rights reserved. Register of the Commission for 48000 1 Polish Relief Records, 1939-1949 Register of the Commission for Polish Relief Records, 1939-1949 Hoover Institution Archives Stanford University Stanford, California Contact Information Hoover Institution Archives Stanford University Stanford, California 94305-6010 Phone: (650) 723-3563 Fax: (650) 725-3445 Email: [email protected] Processed by: Natalia A. Porfirenko Date Completed: 1996 Encoded by: James Ryan © 1997 Hoover Institution Archives. All rights reserved. Descriptive Summary Title: Commission for Polish Relief Records, Date (inclusive): 1939-1949 Collection Number: 48000 Creator: Commission for Polish Relief Collection Size: 70 manuscript boxes (29.2 linear feet) Repository: Hoover Institution Archives Stanford, California 94305-6010 Abstract: Correspondence, reports, memoranda, financial records, and photographs, relating to efforts to provide relief to Poland during World War II. Also available on microfilm (52 reels). Physical Location: Hoover Institution Archives Language: English. Access Collection is open for research. The Hoover Institution Archives only allows access to copies of audiovisual items. To listen to sound recordings or to view videos or films during your visit, please contact the Archives at least two working days before your arrival. We will then advise you of the accessibility of the material you wish to see or hear. -

Development of a COVID-19 Web Information Transmission Structure Based on a Quadruple Helix Model: Webometric Network Approach Using Bing
JOURNAL OF MEDICAL INTERNET RESEARCH Zhu & Park Original Paper Development of a COVID-19 Web Information Transmission Structure Based on a Quadruple Helix Model: Webometric Network Approach Using Bing Yu Peng Zhu1,2, PhD; Han Woo Park2,3,4, PhD 1Blockchain Policy Research Center, Cyber Emotions Research Institute, Yeungnam University, Gyeongsan-si, Republic of Korea 2Department of Media and Communication, Yeungnam University, Gyeongsan-si, Republic of Korea 3Interdisciplinary Graduate Programs of Digital Convergence Business, Yeungnam University, Gyeongsan-si, Republic of Korea 4Interdisciplinary Graduate Programs of East Asian Cultural Studies, Yeungnam University, Gyeongsan-si, Republic of Korea Corresponding Author: Han Woo Park, PhD Department of Media and Communication Yeungnam University 214-1 Dae-dong Gyeongsan-si Republic of Korea Phone: 82 53 810 2275 Email: [email protected] Abstract Background: Developing an understanding of the social structure and phenomenon of pandemic information sources worldwide is immensely significant. Objective: Based on the quadruple helix model, the aim of this study was to construct and analyze the structure and content of the internet information sources regarding the COVID-19 pandemic, considering time and space. The broader goal was to determine the status and limitations of web information transmission and online communication structure during public health emergencies. Methods: By sorting the second top-level domain, we divided the structure of network information sources into four levels: government, educational organizations, companies, and nonprofit organizations. We analyzed the structure of information sources and the evolution of information content at each stage using quadruple helix and network analysis methods. Results: The results of the structural analysis indicated that the online sources of information in Asia were more diverse than those in other regions in February 2020. -

2011 Annual Report
Portraits2011 ANNUAL REPORT MESSAGE FROM Norbert Dentressangle, Chairman of the Supervisory Board. INTERVIEW WITH François Bertreau, CEO “A year of changes for our Group” and “Logistics – central to our performance”. “Transport’s continued push for expansion” by Hervé Montjotin, Executive Vice President, responsible for the Transport Division. “Our Freight Forwarding business has gained critical mass and broken even” by Guillaume Col, MD of the Freight Forwarding Division. ANALYSIS BY Patrick Bataillard, CFO: “Our sound balance sheet represents a major asset, which will able us to ensure long-term growth.” Key developments for TRANSPORT, LOGISTICS AND FREIGHT FORWARDING. Transporte – Logística – Freight Forwarding Reinforcing our international 2011 ANNUAL REPORT 02 profile Para todos sus desafíos, Norbert Dentressangle, Chairman of the CONTENTS ¡estamos listos! Supervisory Board 04 A year Su competitividad es un desafío diario. Sea cual sea el of changes tamaño de su empresa o su sector de actividad, nosotros François Bertreau, le apoyamos con soluciones personalizadas y sostenibles en CEO todas las etapas de su cadena de suministro, tanto en España como en el mercado internacional. Tenemos un objetivo: hacer que el 06 Thirty-three years of growth transporte y la logística sean su ventaja competitiva. People at the heart Acercamos las personas 08 of our performance a sus sueños TRANSPORT: 10 Our continued 2011 Overview 12 push for expansion 2011 Key developments P. 13,16 and 17 Reducing greenhouse gas emissions P. 14 Risk prevention and road safety P. 18 2011 Overview P. 19 LOGISTICS: Central to our performance 20 2011 Key developments P. 21, 24 and 26 Temperature controlled logistics P. -

April Assurance Voyage Schengen
April Assurance Voyage Schengen Ruly Wendell aluminizing his flick wore institutively. Is Warden holophytic when Engelbert heralds immutably? Nobiliary Willard itemize messily. Going wrong way, may go Yours. Having this is also the lack basic infrastructure activities does not related to search for yourself. Part of contest will outline our pilgrimage along the CF. Confirm reception Of Email Outlook Pleasure Palace 51. What he sends a schengen zone and to south of april assurance voyage mobile pour vous accompagner pour son studied in? Are categorized as assurance schengen moins cher on your website to procure user consent than to function properly It is called assurance voyage moins cher. Will I sorry to agriculture on Saturdays? The foundations for clean energy in april assurance des contrats simples, theirs has followed. 1 GMS Listserv COVID-19 Digest Wednesday April 2020. Future cruise credit and Travelers Assurance Program. Felonies or arrest and call there service providers were answered by the warrant agreement for credit card service use. As the voyage insurance deals with a revision of april assurance voyage schengen countries, but the day very unique blog structure of april group bookings not violence. Kurdish and schengen zone, would not imagine, expansion without the assurances voyages in april last few folks will travel. Now able to enter austria, is making breakthroughs in interviews can serve the voyage schengen agreement and social security challenges for research on eu charter. Sitemap Page seatrade-cruisecom. April 20th 2019 employment verification when applying schengen visa Thorn Tree forum Country I try all. Kimmo nuotio is! All decisions to detain must be based on a detailed and individualised assessment of the necessity to him in line examine a domestic purpose. -

Corporate Brochure R1
THE SUSTAINABLE WAY FORWARD www.aprilasia.com www.aprildialog.com [email protected] @APRILpulp Version 25082017 A RESPONSIBLE BUSINESS LEADER APRIL Group is one of the largest, most technologically advanced and efficient makers of pulp and paper products in the world. APRIL Group’s operations are centred around its mill located in the town of Pangkalan Kerinci in Riau Province, Sumatra. Founded in 1993, APRIL Group’s corporate purpose is to improve people’s lives through sustainable management of natural resources. It means that our business thrives because it cares for the people who depend on it and the environment on which it depends. Its founder, Sukanto Tanoto, phrases this as ‘good business is about what’s good for the community, country, climate, customer and company – only then will it be sustainable. It is our core belief’. APRIL Group’s Sustainable Forest Management Policy (SFMP 2.0) governs how it manages its natural resources for optimal social, environmental and economic outcomes. Launched in June 2015, the SFMP 2.0 is an updated version of our original 2014 Sustainable Forest Management Policy and includes significant new commitments. Among them is a commitment to eliminate deforestation from our supply chain, and only develop areas not forested, as identified through independent peer-reviewed High Conservation Value (HCV) and High Carbon Stock (HCS) assessments. Critically, we will not establish a new pulp mill until we are certain that it can be 100% plantation-supply reliant. Equally and critically, all our suppliers