Spirometrosis in Asiatic Lion (Panthera Leo
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
List of Trainees of Egp Training
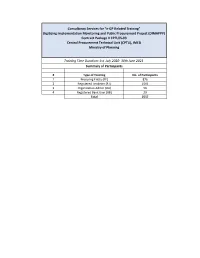
Consultancy Services for “e-GP Related Training” Digitizing Implementation Monitoring and Public Procurement Project (DIMAPPP) Contract Package # CPTU/S-03 Central Procurement Technical Unit (CPTU), IMED Ministry of Planning Training Time Duration: 1st July 2020- 30th June 2021 Summary of Participants # Type of Training No. of Participants 1 Procuring Entity (PE) 876 2 Registered Tenderer (RT) 1593 3 Organization Admin (OA) 59 4 Registered Bank User (RB) 29 Total 2557 Consultancy Services for “e-GP Related Training” Digitizing Implementation Monitoring and Public Procurement Project (DIMAPPP) Contract Package # CPTU/S-03 Central Procurement Technical Unit (CPTU), IMED Ministry of Planning Training Time Duration: 1st July 2020- 30th June 2021 Number of Procuring Entity (PE) Participants: 876 # Name Designation Organization Organization Address 1 Auliullah Sub-Technical Officer National University, Board Board Bazar, Gazipur 2 Md. Mominul Islam Director (ICT) National University Board Bazar, Gazipur 3 Md. Mizanoor Rahman Executive Engineer National University Board Bazar, Gazipur 4 Md. Zillur Rahman Assistant Maintenance Engineer National University Board Bazar, Gazipur 5 Md Rafiqul Islam Sub Assistant Engineer National University Board Bazar, Gazipur 6 Mohammad Noor Hossain System Analyst National University Board Bazar, Gazipur 7 Md. Anisur Rahman Programmer Ministry Of Land Bangladesh Secretariat Dhaka-999 8 Sanjib Kumar Debnath Deputy Director Ministry Of Land Bangladesh Secretariat Dhaka-1000 9 Mohammad Rashedul Alam Joint Director Bangladesh Rural Development Board 5,Kawranbazar, Palli Bhaban, Dhaka-1215 10 Md. Enamul Haque Assistant Director(Construction) Bangladesh Rural Development Board 5,Kawranbazar, Palli Bhaban, Dhaka-1215 11 Nazneen Khanam Deputy Director Bangladesh Rural Development Board 5,Kawranbazar, Palli Bhaban, Dhaka-1215 12 Md. -

Acknowledgement
Acknowledgement The author is ever grateful and indebted to the Almighty God without whose grace it would have never been possible to pursue this study in this field of science and to complete this production report writing for the Degree of Doctor of Veterinary Medicine (DVM). The author would like to thank his reverend and beloved teacher and Supervisor Md. Emran Hossain,Associate Professor Department of Animal Science Chittagong Veterinary and Animal Sciences, Chittagong for his scholastic guidance, uncompromising principles, sympathetic supervision, valuable advice and constant inspiration of this study and preparing the manuscript. The author would like to express his deed sense of gratitude and heartfelt appreciation to Dr. Md. M Morshed Chowdury,veterinary officer cum Curator and Dr. Shahadat Hossain, Shuvo, Educatioal officer cum veterinarian, Chittagong Zoo, for their cordial help at Chittagong Zoo. The author would like to express his sincere gratitude and gratefulness to Dr. Mostafizur Rahman, Veterinary surgeon, Bongobondhu Sheikh Mujibur Rahman safari park, Dulhazra, Cox’s-bazar for his valuable suggestion and cordial help for completion of the report work. The Author would like to thank his sincere gratitude and gratefulness to DR. Anwar shahadat, Scientific officer , Bangladesh National zoo for his cordial help. --------------------------------------- The Author 1 List of table… Table No Contents Page 1 Feeding practice (in gram) per at J.N Dairy Farm at Bohardarhat, 11 Chittagong 2 Feeding practice (in gram) per deer at National Zoo, Dhaka 12 3 Feeding practice (in gram) per deer at Bongobondhu sheikh 13 Mujibur rahman safari park, Dulhazara, Cox’s Bazar. 4 Feeding Budgeting of Spotted deer (Azad el al, 2005) 14 5 Recommended feed items for spotted (Azad et al, 2005) 14 6 The no of the male and female ratio 15 7 Schedule of day-to-day operation on spotted deer premises (Azad et 16 al, 2005) List of figure…. -

Giraffa Camelopardalis)
Bangladesh Journal of Veterinary and Animal Sciences, Vol. 9, No. 1, January - June 2021 Bangladesh Journal of Veterinary and Animal Sciences pISSN 2227-6416 Journal home page: www.bjvas.com eISSN 2709-2542 Research article Morphology and morphometric analysis of bones of the forelimb of giraffe (Giraffa camelopardalis) Sadia Jahan1, Md. Shahriar Hasan Sohel2and Mohammad Lutfur Rahman1* 1Department of Anatomy & Histology, Chattogram Veterinary and Animal Sciences University, Bangladesh. 2Laboratory of Veterinary Anatomy, Joint Graduate School of Veterinary Sciences, Gifu University, Japan. A R T I C L E I N F O A B S T R A C T We studied the bones of forelimb of male giraffe (Giraffa Article history: camelopardalis) to record the gross anatomical and morphometrical Received: 10/05/2021 features of the scapula, humerus, radius and ulna. We observed some unique anatomical features that will be helpful for radiographic Accepted: 28/06/2021 interpretation and forensic investigations. For this purpose all the bones of thoracic limb were collected timely from the burial ground, identified Keywords: by their morphological features and finally measured after processing with chemicals. The scapula was a triangular flat bone and the lateral Morphology and surface of scapula was unequally divided into supraspinous (fossa morphometry, giraffe, thoracic supraspinata) and infraspinous fossa (fossa infraspinata) by a well- limb, scapula, humerus, radius developed spine (spina scapulae). The humerus was a major and ulna, skeleton massive bone in the appendicular skeleton to bear the total body weight. The average length of humerus was 56.17 cm that run from the shoulder to the elbow. It possessed a cylindrical diaphysis which was somewhat *Corresponding author: compressed laterally and two enlarged epiphysis namely-proximal epiphysis and distal epiphysis. -

Final 2012 June ZPM Full Text
Dhaka Zoo ... now Bangladesh National Zoo ... a casual inspection Sally Walker Background Various enclosures There is no enrichment inside My 10th visit to Dhaka Zoo was anywhere. The animals don’t get much inspired by the news that Dhaka Zoo Crocodiles time outside where they have to go had ordered another large The Crocodilian enclosures are one at a time. The one lion observed consignment of animals from South relatively new but the style dates way as close quarters was without spirit Africa. A couple of years ago the zoo back: concrete, chain mesh, very little and in indifferent condition. had ordered such a consignment and I water, etc. One is acceptable with a was informed that many of the animals large tank and an area for sunning with died in a relatively short time. I flew grass and sand. The Crocodiles and over to Dhaka shortly after hearing this Gharials are said to be breeding, news and it was confirmed by according to the Curator. One of the management that that was the case. A enclosures has protective sides two few weeks later virtually all of them feet in the ground but the crocs dig but one or two had died. It is hard to large holes and two feet may not be get information straight in such cases sufficient to keep them from tunneling as the management running the zoo at out. A great deal of improvement is that time, is long gone by now and the required for these aquatic reptiles...in new management denied the previous husbandry, in enclosure design, in deaths. -

Bangladesh DR
Wildlife policy in Bangladesh DR. NAZMUL HODA Zoo Veterinarian , Bangladesh National Zoo Department of Livestock Services Ministry of Fisheries and Livestock, Bangladesh. Virtual Meeting on Wildlife Health for OIE Members in South Asia Wednesday 13th January 2021 Wildlife policy Act : Wildlife (Protection and Security) Act 2012 Rules : 1. Pet Bird Management Rules 2020 2. Crocodile Rearing Rules 2019 3. Deer and Elephant Rearing Rules 2017 ◦ Ministry of Fisheries and Livestock regulates Animal Health related issues. Wildlife disease investigation is made in the laboratories of Department of Livestock Services and BLRI as well. ◦ Ministry of Environment , Forest and Climate Change is going to establish an wildlife disease diagnostic laboratory and other steps for wildlife disease surveillance. Till now MoEFCC has no role on wildlife disease investigation. Activities relating to wildlife Veterinary Services relating to wildlife ◦ clinical care of wildlife 1. Veterinarians of DLS are associated with clinical care of wild animals all over the country. 2. Rescue of wild animals in collaboration with forest department. 3. Bangladesh National Zoo and Rangpur Zoo has wild animal Hospitals and Skill manpower for wildlife treatment. 4. Immunization of Captive wild animals. 5. Wildlife disease diagnosis. Disease reporting Summary of key diseases reported in wildlife in the past year Central Disease Investigation Laboratories (CDIL) has analysed wildlife samples from both sick and dead animals. In the past year (2020) we have reported tuberculosis, New Castlte Disease, Mycoplasmosis, Pasteurellosis, Coli-enteritis, Chlostridial Enteritis, Dermatitis, Mange, Ring Worm , Tape worm infestation, Cryptosporidiosis, Giardiasis,Coccidiosis and Capture Myopathy . One Health Outline how wildlife are included in One Health activities ◦ In Bangladesh there is an One Health Secretariat where three government officials are working from DLS, Forest Department and Department Health Services. -

Bangladesh Zoo Seeks Mate for Lonely Kanchi the Rhino 15 January 2021, by Shafiqul Alam
Bangladesh zoo seeks mate for lonely Kanchi the Rhino 15 January 2021, by Shafiqul Alam "Her mood swings frequently. Sometimes she does not respond to my calls. It is mainly because she has grown up alone all these years," Mia said. "I tell her that we will soon find her a male partner. But she is restless. She needs a partner desperately." Abdul Latif, curator of the zoo, said the coronavirus pandemic had blocked recent efforts to bring in a male rhino from Africa. "We know she feels lonely and we are trying our best to buy a suitable partner," Latif told AFP. Kanchi the rhino at a Bangladeshi zoo has been alone since the death of her partner in 2014 A lonely rhinoceros at a Bangladesh zoo is looking for new love after losing her partner seven years ago, but pandemic travel restrictions are hampering her keeper's attempts to play matchmaker. Kanchi, a star attraction at the Bangladesh National Zoo, is at her most fertile age. But since the death of her male partner in 2014, she has been living on her own in her muddy pen in the northern suburb of the capital. The Bangladesh National Zoo said the pandemic had blocked recent efforts to bring in a male rhino from Africa The malaise of Kanchi the rhino has become for Kanchi increasingly apparent to the two million-plus visitors a year at the Dhaka landmark. Kanchi refuses food and often snubs her carer There has been more attention given to Kanchi, Farid Mia, who hugs the rhino and scratches its however, since the plight of Kaavan, the world's neck and shoulders. -
Bangladesh Tropical Forests and Biodiversity Assessment
BANGLADESH TROPICAL FORESTS AND BIODIVERSITY ASSESSMENT MAY 2016 May 2016 This publication was produced for review by the United States Agency for International Development. It was prepared by Integra LLC BANGLADESH TROPICAL FORESTS AND BIODIVERSITY ASSESSMENT UNITED STATES FOREIGN ASSISTANCE ACT, SECTION 118/119 REPORT MAY 2016 Report Authors: Patricia Foster-Turley, Rishiraj Das, Md. Kamrul Hasan, Peerzadi Rumana Hossain Prepared for USAID Bangladesh Prepared under the Restoring the Environment through Prosperity, Livelihoods and Conserving Ecosystems (REPLACE) Contract, Award Number AID-388-TO-16-00001 Integra Government Services International 1100 Vermont Avenue NW, Suite 750 Washington, DC 20005 +1 202 898 4110 www.integrallc.com DISCLAIMER The author’s views expressed in this publication do not necessarily reflect the views of the United States Agency for International Development or the United States Government. i USAID BANGLADESH TROPICAL FORESTS AND BIODIVERSITY ASSESSMENT TABLE OF CONTENTS LIST OF ACRONYMS IV! I. EXECUTIVE SUMMARY 1! II. INTRODUCTION 4! PURPOSE 4! METHODS 4! REPORT LAYOUT 5! III. ACTIONS NECESSARY TO CONSERVE TROPICAL FORESTS AND BIODIVERSITY IN BANGLADESH 6! A. SOCIAL, ECONOMIC AND POLITICAL CONTEXT 6! B. STATUS OF TROPICAL FORESTS AND BIODIVERSITY 7! C. GOVERNMENT AND LEGAL FRAMEWORK 13! D. KEY PROGRAMS ON BIODIVERSITY AND FORESTS 20! E. DIRECT THREATS TO BIODIVERSITY AND FORESTS 24! F. INDIRECT THREATS TO FORESTS AND BIODIVERSITY 27! G. ACTIONS NECESSARY TO CONSERVE BIODIVERSITY AND FORESTS 31! IV. ANALYSIS OF USAID BANGLADESH CDCS IN CONTEXT OF TROPICAL FOREST AND BIODIVERSITY NEEDS 36! A. OVERVIEW 36! ii May 2016 B. ENVIRONMENT, CLIMATE CHANGE AND ENERGY PROGRAMS 37! C. OTHER USAID OFFICES 40! D. -

Occurrence of Gastrointestinal Helminths in Captive Rhesus Macaques (Macaca Mulatta)
Bangladesh J. Zool. 46(2): 231-237, 2018 ISSN: 0304-9027 (print) 2408-8455 (online) OCCURRENCE OF GASTROINTESTINAL HELMINTHS IN CAPTIVE RHESUS MACAQUES (MACACA MULATTA) Taniza Tabasshum*, Mandira Mukutmoni and Aleya Begum Department of Zoology, University of Dhaka, Dhaka-1000, Bangladesh Abstract: A total of 66 (sixty-six) fresh fecal samples were collected during July 2017 to October 2018 from rhesus macaques (Macaca mulatta) residing in Bangladesh National Zoo, Dhaka. Samples were analyzed applying formol-ether concentration technique. All the rhesus macaques, irrespective of age and sex, were found to be infested with one or more species of gastrointestinal (GI) helminth parasites. Ascaris spp. was noticed in all the fecal samples. Overall intensity of helminths was higher in male (38.53) that in female (31.04) comprising the maximum (58.08) in adult male (p < 0.005). The highest intensity was of Ascaris spp. (3.33 ± 1.39) and found in adult male hosts. Young female rhesus macaques displayed the maximum intensity of Trichuris spp. (3.56 ± 0.73). Key words: Captive, rhesus macaques, helmench, prevalence, intensity INTRODUCTION Non-human primates (NHP) are the closest living biological relatives, offering critical perceptions into human evolution, biology and behavior and playing important roles in the livelihoods, cultures, and religions of many societies. They contribute to tropical biodiversity and run many functions of ecological importance. Among the NHPs, rhesus macaques (Macaca mulatta) are synanthropic, thriving in human altered environments that help them to be among the most widely distributed and successful primates in the world (Hasan et al. 2013). But unsustainable human activities are now the major force driving primate species to extinction (Estrada et al. -

Bangladesh Vulture Conservation Action Plan 2016-2025
Bangladesh Vulture Conservation Action Plan 2 0 1 6 - 2 0 2 5 ���������������������� A B M Sarowar Alam Khadija Rawshan Tarik Kabir Sakib Ahmed Ashit Ranjan Paul Khorsheda Yasmeen M. Monirul H. Khan Ishtiaq Uddin Ahmad Design: Jahangir Alam Copyright: © Ministry of Environment and Forests, Government of the People’s Republic of Bangladesh Citation: MoEF, 2016. Bangladesh Vulture Conservation Action Plan 2016-2025. Ministry of Environment and Forests, Government of the People’s Republic of Bangladesh, Dhaka, Bangladesh. pp. X+58. Cover Photo: © Enam Ul Haque Printing: Shoilpik Choa ISBN: 978-984-34-1222-5 FOREWORD Bangladesh has a record of seven vulture species but unfortunately, the vulture population of Bangladesh has went through a catastrophic decline similar to the decline seen across South Asia. Overwhelming evidence from myriad scientific literatures confirm that veterinary drug diclofenac (a Non-steroidal anti-inflammatory drug or NSAID), widely prescribed and used for cattle treatment in South Asia, is responsible for the drastic drop of vulture population. Taking account of the evidence, Government of Bangladesh, in effort to save the vultures, has banned the production, distribution and sale of veterinary diclofenac in 2010, and later declared two Vulture Safe Zones (VSZ-1: 19,663.18 km2 and VSZ-2: 27717.26 km2) in 2014 - both decisions reflect the state’s commitment to vulture conservation. Regrettably, diclofenac is still available at border areas of Bangladesh; moreover other NSAIDs like ketoprofen and flunixin have been found to be toxic to Gyps vultures. Additionally, habitat loss and food shortage have always been a threat to the vulture population in Bangladesh. -

India Starts Vaccine Drive As Global Deaths Top 2 Million
JAMADA ALTHANI 4, 1442 AH SUNDAY, JANUARY 17, 2021 16 Pages Max 22º Min 07º 150 Fils Established 1961 ISSUE NO: 18336 The First Daily in the Arabian Gulf www.kuwaittimes.net Abbas announces Palestinian Talal Al-Ajmi Al-Danah grand Desert off-roading final Historic divides fuel Man 5 elections for May and July 10 prize winner of KD 1,500,000 13 frontier for Qatar women 15 United, Liverpool rivalry India starts vaccine drive as global deaths top 2 million Lebanon hits record coronavirus deaths, infections NEW DELHI: India kicked off one of the world’s worst-funded healthcare systems. Regular child largest coronavirus vaccination drives yesterday as inoculations are a “much smaller game” and vacci- the pandemic spread at a record pace and global nating against COVID-19 will be “deeply challeng- COVID-19 deaths surged past two million. The ing”, said Satyajit Rath from India’s National World Health Organization has called for accelerat- Institute of Immunology. ing vaccine rollouts worldwide as well as ramping up The government has readied tens of thousands of efforts to study the sequencing of the virus, which refrigeration tools and about 150,000 specially has infected more than 93 million people globally trained staff to try and overcome some of those since it was first detected in China in late 2019. challenges. The vaccines will also have high securi- India, home to 1.3 billion people, has the world’s ty, so that doses do not end up being sold on India’s second-largest caseload. The government has given large black market for medicines. -

Issue Full File
Journal of Tourismology Volume: 6 • Number: 1 • June 2020 e-ISSN: 2459-1939 • DOI: 10.26650/jot Journal of Tourismology is the official peer-reviewed, international journal of the Istanbul University Faculty of Economics Authors bear responsibility for the content of their published articles. Owner Istanbul University, Faculty of Economics EDITORIAL MANAGEMENT Editor-in-Chiefs Mehmet Erkan (Co-Editor, Prof., Istanbul University, Istanbul, Turkey) Fusun Istanbullu Dincer (Editor-in-Chief, Prof., Istanbul University, Istanbul, Turkey) Gurel Cetin (Managing Editor, Assoc. Prof., Istanbul University, Istanbul, Turkey) Production Editors Ibrahim Cifci (Res. Asst., Istanbul University, Department of Tourism Management, Turkey E-mail: [email protected]) Mehmet Altug Sahin (Res. Asst., Istanbul University, Department of Tourism Management, Turkey E-mail: [email protected]) Mert Ogretmenogku (Res. Asst., Istanbul University, Department of Tourism Management, Turkey E-mail: [email protected]) Methodology Editor Hossein Olya (Dr., Tourism Management, Faculty of Business, Oxford Brookes University, UK E-mail: [email protected]) English Language Editor Elizabeth Mary Earl, İstanbul University, School of Foreign Languages (English) E-mail: [email protected] Alan James Newson, İstanbul University, School of Foreign Languages (English) E-mail: [email protected] EDITORIAL BOARD Faizan Ali (Asst. Prof.), Florida State University, USA. Email: [email protected] Amir Shani (PhD), Ben-Gurion University of The Negev, Israel. Email: [email protected] Arta Antonovica (Profesor Contratado Doctor), Universitad Rey Juan Carlos, Spain. Email: [email protected] Ashish Dahiya (Prof.), GD Goenka University, India. Email: [email protected] Dimitri Iaonnides (Prof.), Meet Mid Sweden University, Sweden. -

Expression of Interest
BANGLADESH NATIONAL ZOO Preparation of master Plan for Bangladesh National Zoo, Dhaka and Rangpur recreation garden and Zoo. Expression of Interest For Preparation of master Plan for Bangladesh National Zoo, Dhaka and Rangpur recreation garden and Zoo. January, 2019 1 Notice of EOI Government of People's Republic of Bangladesh Project Director- Master Plan Preparation of Bangladesh National Zoo & Rangpur Zoo and Inevitable Infrastructure Repairing & Development Project and Curator, Bangladesh National Zoo, Mirpur, Dhaka. Memo No. 33.01.0000.106.01.035.17-168 Date: 24-01-2019 Request for Expression of Interest (EOI) 1 Ministry/Division Ministry of Fisheries and Livestock 2 Agency Department of Livestock Services Dr. S.M. Nazrul Islam, Project Director- Master Plan Preparation of Bangladesh 3 Procuring Entity Name National Zoo & Rangpur Zoo and Inevitable Infrastructure Repairing & Development Project and Curator, Bangladesh National Zoo, Mirpur, Dhaka. 4 Procuring Entity District Dhaka Hiring of consulting firm for preparation of Master Plan for 5 Invitation for Modernization of Bangladesh National Zoo, Dhaka and Rangpur Zoo. 6 Invitation Ref No. 33.01.0000.106.01.035.17-168 7 Date Date: 24/01/2019 KEY INFORMATION 8 Procurement Method OTM (National/International)/EOI- on QCBS FUNDING INFORMATION 9 Source of Funds Development Budget (GOB) Development Partners (if 10 N/A applicable) PARTICULAR INFORMATION 11 Project/Program Code 224253600 Preparation of Master Plan for Modernization of Bangladesh 12 Project/Program Name National Zoo, Dhaka and Rangpur Zoo. Date: 24/02/2019 Time: Up to Office hour. EOI Closing Date, Time & 13 Place: Project Director and Curator’s Office, Bangladesh Place National Zoo, Mirpur, Dhaka.