Download Fulltext
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Linearized Esculentin-2EM Shows Ph Dependent Antibacterial Activity With
Molecular and Cellular Biochemistry https://doi.org/10.1007/s11010-021-04181-7 Linearized esculentin‑2EM shows pH dependent antibacterial activity with an alkaline optimum Erum Malik1 · David A. Phoenix2 · Timothy J. Snape3 · Frederick Harris4 · Jaipaul Singh4 · Leslie H. G. Morton4 · Sarah R. Dennison5 Received: 5 November 2020 / Accepted: 12 May 2021 © The Author(s) 2021 Abstract Here the hypothesis that linearized esculentin 2EM (E2EM-lin) from Glandirana emeljanovi possesses pH dependent activ- ity is investigated. The peptide showed weak activity against Gram-negative bacteria (MLCs ≥ 75.0 μM) but potent efcacy towards Gram-positive bacteria (MLCs ≤ 6.25 μM). E2EM-lin adopted an α-helical structure in the presence of bacterial membranes that increased as pH was increased from 6 to 8 (↑ 15.5–26.9%), whilst similar increases in pH enhanced the ability of the peptide to penetrate (↑ 2.3–5.1 mN m−1) and lyse (↑ 15.1–32.5%) these membranes. Theoretical analysis predicted that this membranolytic mechanism involved a tilted segment, that increased along the α-helical long axis of E2EM-lin (1–23) in the N → C direction, with − < µH > increasing overall from circa − 0.8 to − 0.3. In combination, these data showed that E2EM-lin killed bacteria via novel mechanisms that were enhanced by alkaline conditions and involved the formation of tilted and membranolytic, α-helical structure. The preference of E2EM-lin for Gram-positive bacteria over Gram-negative organisms was primarily driven by the superior ability of phosphatidylglycerol to induce α-helical structure in the peptide as compared to phosphatidylethanolamine. These data were used to generate a novel pore-forming model for the membranolytic activity of E2EM-lin, which would appear to be the frst, major reported instance of pH dependent AMPs with alkaline optima using tilted structure to drive a pore-forming process. -

Parasitic Nematodes of Pool Frog (Pelophylax Lessonae) in the Volga Basin
Journal MVZ Cordoba 2019; 24(3):7314-7321. https://doi.org/10.21897/rmvz.1501 Research article Parasitic nematodes of Pool Frog (Pelophylax lessonae) in the Volga Basin Igor V. Chikhlyaev1 ; Alexander B. Ruchin2* ; Alexander I. Fayzulin1 1Institute of Ecology of the Volga River Basin, Russian Academy of Sciences, Togliatti, Russia 2Mordovia State Nature Reserve and National Park «Smolny», Saransk, Russia. *Correspondence: [email protected] Received: Febrary 2019; Accepted: July 2019; Published: August 2019. ABSTRACT Objetive. Present a modern review of the nematodes fauna of the pool frog Pelophylax lessonae (Camerano, 1882) from Volga basin populations on the basis of our own research and literature sources analysis. Materials and methods. Present work consolidates data from different helminthological works over the past 80 years, supported by our own research results. During the period from 1936 to 2016 different authors examined 1460 specimens of pool frog, using the method of full helminthological autopsy, from 13 regions of the Volga basin. Results. In total 9 nematodes species were recorded. Nematode Icosiella neglecta found for the first time in the studied host from the territory of Russia and Volga basin. Three species appeared to be more widespread: Oswaldocruzia filiformis, Cosmocerca ornata and Icosiella neglecta. For each helminth species the following information included: systematic position, areas of detection, localization, biology, list of definitive hosts, the level of host-specificity. Conclusions. Nematodes of pool frog, excluding I. neglecta, belong to the group of soil-transmitted helminthes (geohelminth) and parasitize in adult stages. Some species (O. filiformis, C. ornata, I. neglecta) are widespread in the host range. -

Diet Composition of the Karpathos Marsh Frog (Pelophylax Cerigensis): What Does the Most Endangered Frog in Europe Eat?
Animal Biodiversity and Conservation 42.1 (2019) 1 Diet composition of the Karpathos marsh frog (Pelophylax cerigensis): what does the most endangered frog in Europe eat? P. Pafilis, G. Kapsalas, P. Lymberakis, D. Protopappas, K. Sotiropoulos Pafilis, P., Kapsalas, G., Lymberakis, P., Protopappas, D., Sotiropoulos, K., 2019. Diet composition of the Karpathos marsh frog (Pelophylax cerigensis): what does the most endangered frog in Europe eat? Animal Biodiversity and Conservation, 42.1: 1–8, https://doi.org/10.32800/abc.2019.42.0001 Abstract Diet composition of the Karpathos marsh frog (Pelophylax cerigensis): what does the most endangered frog in Europe eat? The Karpathos marsh frog (Pelophylax cerigensis) is considered the most endangered frog in Europe. Here we assess its feeding ecology and examine 76 individuals from the two known populations using the stomach flushing method. We also measured body weight, snout–vent length, mouth width and prey width and length. Pelophylax cerigensis follows the feeding pattern of green frogs of the adjacent areas, with Coleoptera, Araneae, Isopoda and Hymenoptera being the main prey groups. The two populations differed in body size but had similar values of prey abundance and frequency. It seems that P. cerigensis follows a strict feeding strategy. Further research on prey availability in its habitats will provide valuable insight. Key words: Diet, Endangered species, Islands, Frogs, Mediterranean Resumen Composición de la dieta de la rana de Kárpatos (Pelophylax cerigensis): ¿qué come la rana más amenazada de Europa? La rana de Kárpatos (Pelophylax cerigensis) es considerada la rana más amenazada de Europa. Aquí evaluamos su ecología alimentaria y examinamos 76 individuos de las dos poblaciones conocidas usando el método del lavado de estómago. -

Pool Frog (Pelophylax Lessonae) Camerano 1882 (Anura, Ranidae), an Addition to the Finnish Amphibian Fauna
Memoranda Soc. Fauna Flora Fennica 89: 25–31. 2013 25 Pool frog (Pelophylax lessonae) CAMERANO 1882 (Anura, Ranidae), an addition to the Finnish amphibian fauna Tom Hoogesteger, Joel Rahkonen & Ari Karhilahti Hoogesteger, T., Department of Biological and Environmental Science, FI-40014 University of Jyväskylä, Finland. E-mail: [email protected] Rahkonen, J., Department of Biological and Environmental Science, FI-40014 University of Jyväskylä, Finland. E-mail: [email protected] Karhilahti, A., Zoological Museum, FI-20014 University of Turku, Finland. E-mail: [email protected] A population of pool frogs (Pelophylax lessonae) has been discovered in the municipality of Kaari- na, southwestern Finland. The species had not previously been recorded from Finland. The frogs show the external characteristics of the northern clade of the species, which suggests that they are of different origin than the allochthonous edible frogs (Pelophylax kl. esculentus) that are also present in southwestern Finland. Introduction is the case in Denmark and southernmost Swe- den, where P. kl. esculentus is common but nei- In the northern and central parts of Europe, the ther of the parental species is present (Gasc et al. green frog complex consists of two species: the 1997, Fog et al. 2001, Christiansen et al. 2005). marsh frog (Pelophylax ridibundus) and the pool Ridibundus – esculentus -systems, in which P. frog (P. lessonae), as well as the hybrid between kl. esculentus reproduces with P. ridibundus, are these two species, the edible frog (P. kl. esculen- known from Germany, the Baltic coast of Poland tus). The hybrids reproduce by means of hybri- and the island of Bornholm, Denmark (Rybacki dogenesis, in which one parental genome is com- & Fog 1995, Fog et al. -

Standard Common and Current Scientific Names for North American Amphibians, Turtles, Reptiles & Crocodilians
STANDARD COMMON AND CURRENT SCIENTIFIC NAMES FOR NORTH AMERICAN AMPHIBIANS, TURTLES, REPTILES & CROCODILIANS Sixth Edition Joseph T. Collins TraVis W. TAGGart The Center for North American Herpetology THE CEN T ER FOR NOR T H AMERI ca N HERPE T OLOGY www.cnah.org Joseph T. Collins, Director The Center for North American Herpetology 1502 Medinah Circle Lawrence, Kansas 66047 (785) 393-4757 Single copies of this publication are available gratis from The Center for North American Herpetology, 1502 Medinah Circle, Lawrence, Kansas 66047 USA; within the United States and Canada, please send a self-addressed 7x10-inch manila envelope with sufficient U.S. first class postage affixed for four ounces. Individuals outside the United States and Canada should contact CNAH via email before requesting a copy. A list of previous editions of this title is printed on the inside back cover. THE CEN T ER FOR NOR T H AMERI ca N HERPE T OLOGY BO A RD OF DIRE ct ORS Joseph T. Collins Suzanne L. Collins Kansas Biological Survey The Center for The University of Kansas North American Herpetology 2021 Constant Avenue 1502 Medinah Circle Lawrence, Kansas 66047 Lawrence, Kansas 66047 Kelly J. Irwin James L. Knight Arkansas Game & Fish South Carolina Commission State Museum 915 East Sevier Street P. O. Box 100107 Benton, Arkansas 72015 Columbia, South Carolina 29202 Walter E. Meshaka, Jr. Robert Powell Section of Zoology Department of Biology State Museum of Pennsylvania Avila University 300 North Street 11901 Wornall Road Harrisburg, Pennsylvania 17120 Kansas City, Missouri 64145 Travis W. Taggart Sternberg Museum of Natural History Fort Hays State University 3000 Sternberg Drive Hays, Kansas 67601 Front cover images of an Eastern Collared Lizard (Crotaphytus collaris) and Cajun Chorus Frog (Pseudacris fouquettei) by Suzanne L. -

Parasitic Nematodes of Pool Frog (Pelophylax Lessonae) in the Volga Basin
Revista MVZ Córdoba ISSN: 0122-0268 ISSN: 1909-0544 [email protected] Universidad de Córdoba Colombia Parasitic nematodes of Pool Frog (Pelophylax lessonae) in the Volga Basin V. Chikhlyaev, Igor; B. Ruchin, Alexander; I. Fayzulin, Alexander Parasitic nematodes of Pool Frog (Pelophylax lessonae) in the Volga Basin Revista MVZ Córdoba, vol. 24, no. 3, 2019 Universidad de Córdoba, Colombia Available in: http://www.redalyc.org/articulo.oa?id=69360322014 DOI: https://doi.org/10.21897/rmvz.1501 This work is licensed under Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International. PDF generated from XML JATS4R by Redalyc Project academic non-profit, developed under the open access initiative Revista MVZ Córdoba, 2019, vol. 24, no. 3, September-December, ISSN: 0122-0268 1909-0544 Original Parasitic nematodes of Pool Frog (Pelophylax lessonae) in the Volga Basin Parásitos nematodos de la rana de piscina (Pelophylax lessonae) en la cuenca del Río Volga Igor V. Chikhlyaev DOI: https://doi.org/10.21897/rmvz.1501 Institute of Ecology of the Volga River Basi, Rusia Redalyc: http://www.redalyc.org/articulo.oa?id=69360322014 [email protected] http://orcid.org/0000-0001-9949-233X Alexander B. Ruchin Mordovia State Nature Reserve and National Park , Rusia [email protected] http://orcid.org/0000-0003-2653-3879 Alexander I. Fayzulin Institute of Ecology of the Volga River Basi, Rusia [email protected] http://orcid.org/0000-0002-2595-7453 Received: 04 February 2019 Accepted: 08 July 2019 Published: 29 August 2019 Abstract: Objetive. Present a modern review of the nematodes fauna of the pool frog Pelophylax lessonae (Camerano, 1882) from Volga basin populations on the basis of our own research and literature sources analysis. -
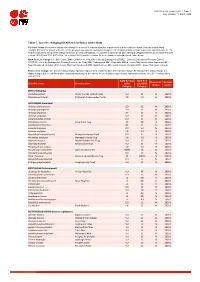
Table 7: Species Changing IUCN Red List Status (2018-2020)
IUCN Red List version 2020-1: Table 7 Last Updated: 19 March 2020 Table 7: Species changing IUCN Red List Status (2018-2020) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2019 (IUCN Red List version 2019-3) and 2020 (IUCN Red List version 2020-1) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered [CR(PE) - Critically Endangered (Possibly Extinct), CR(PEW) - Critically Endangered (Possibly Extinct in the Wild)], EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.); E - Previous listing was an Error. IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2019) List (2020) change version Category -

Sex Reversal Induced by Steroid Hormones in Glandirana Rugosa Frogs
Central JSM Sexual Medicine Mini Review *Corresponding author Masahisa Nakamura, Waseda Research Institute for Science and Engineering, 3-4-1 Okubo, Shinjuku-ku, Waseda University, Tokyo, 169-8555, Japan, Email: Sex Reversal Induced by Steroid [email protected] Submitted: 01 September 2020 Hormones in Glandirana rugosa Accepted: 15 September 2020 Published: 17 September 2020 ISSN: 2578-3718 Frogs Copyright © 2020 Nakamura M, et al. 1,2 2 2 Masahisa Nakamura *, Akira Oike , and Etsuro Ito OPEN ACCESS 1Waseda Research Institute for Science and Engineering, Waseda University, Japan 2Department of Biology, Waseda University, Japan Abstract In general, sex is determined at fertilization of zygotes by sex chromosome composition; this is known as genotypic sex determination in many vertebrate species. Interestingly, steroid hormones can reverse sex of many species in fish, amphibians and reptiles; androgens induce the female-to-male sex reversal, whereas estrogens cause the male-to-female one. For such sex reversal, a functioning sex-determining gene on the sex chromosome is not required. However, little is known about the mechanisms involved in the sex-reversal at histological and molecular levels. To clarify the mechanism of sex reversal, it is very important to detect the first signs of histological changes in the sex-reversing gonads. For this purpose, we have determined a threshold dosage of steroid hormones to induce sex reversal. When tadpoles of Glandirana (G.) rugosa are reared in water containing a threshold dosage of steroid hormones, genetic females and males form a mixture of testis and ovary, the so-called ovotestis during the transit period of sex reversal. -

1704632114.Full.Pdf
Phylogenomics reveals rapid, simultaneous PNAS PLUS diversification of three major clades of Gondwanan frogs at the Cretaceous–Paleogene boundary Yan-Jie Fenga, David C. Blackburnb, Dan Lianga, David M. Hillisc, David B. Waked,1, David C. Cannatellac,1, and Peng Zhanga,1 aState Key Laboratory of Biocontrol, College of Ecology and Evolution, School of Life Sciences, Sun Yat-Sen University, Guangzhou 510006, China; bDepartment of Natural History, Florida Museum of Natural History, University of Florida, Gainesville, FL 32611; cDepartment of Integrative Biology and Biodiversity Collections, University of Texas, Austin, TX 78712; and dMuseum of Vertebrate Zoology and Department of Integrative Biology, University of California, Berkeley, CA 94720 Contributed by David B. Wake, June 2, 2017 (sent for review March 22, 2017; reviewed by S. Blair Hedges and Jonathan B. Losos) Frogs (Anura) are one of the most diverse groups of vertebrates The poor resolution for many nodes in anuran phylogeny is and comprise nearly 90% of living amphibian species. Their world- likely a result of the small number of molecular markers tra- wide distribution and diverse biology make them well-suited for ditionally used for these analyses. Previous large-scale studies assessing fundamental questions in evolution, ecology, and conser- used 6 genes (∼4,700 nt) (4), 5 genes (∼3,800 nt) (5), 12 genes vation. However, despite their scientific importance, the evolutionary (6) with ∼12,000 nt of GenBank data (but with ∼80% missing history and tempo of frog diversification remain poorly understood. data), and whole mitochondrial genomes (∼11,000 nt) (7). In By using a molecular dataset of unprecedented size, including 88-kb the larger datasets (e.g., ref. -

Predatory Ecology of the Invasive Wrinkled Frog (Glandirana Rugosa) in Hawai´I
Gut check: predatory ecology of the invasive wrinkled frog (Glandirana rugosa) in Hawai´i By Melissa J. Van Kleeck and Brenden S. Holland* Abstract Invertebrates constitute the most diverse Pacific island animal lineages, and have correspondingly suffered the most significant extinction rates. Losses of native invertebrate lineages have been driven largely by ecosystem changes brought about by loss of habitat and direct predation by introduced species. Although Hawaii notably lacks native terrestrial reptiles and amphibians, both intentional and unintentional anthropogenic releases of herpetofauna have resulted in the establishment of more than two dozen species of frogs, toads, turtles, lizards, and a snake. Despite well-known presence of nonnative predatory species in Hawaii, ecological impacts remain unstudied for a majority of these species. In this study, we evaluated the diet of the Japanese wrinkled frog, Glandirana rugosa, an intentional biocontrol release in the Hawaiian Islands in the late 19th century. We collected live frogs on Oahu and used museum collections from both Oahu and Maui to determine exploited diet composition. These data were then compared to a published dietary analysis from the native range in Japan. We compiled and summarized field and museum distribution data from Oahu, Maui, and Kauai to document the current range of this species. Gut content analyses suggest that diet composition in the Hawaiian Islands is significantly different from that that in its native Japan. In the native range, the dominant taxonomic groups by volume were Coleoptera (beetles), Lepidoptera (moths, butterflies) and Formicidae (ants). Invasive frogs in Hawaii exploited mostly Dermaptera (earwigs), Amphipoda (landhoppers) and Hemiptera (true bugs). -

Larval Systematics of the Peninsular Malaysian Ranidae (Amphibia: Anura)
LARVAL SYSTEMATICS OF THE PENINSULAR MALAYSIAN RANIDAE (AMPHIBIA: ANURA) LEONG TZI MING NATIONAL UNIVERSITY OF SINGAPORE 2005 LARVAL SYSTEMATICS OF THE PENINSULAR MALAYSIAN RANIDAE (AMPHIBIA: ANURA) LEONG TZI MING B.Sc. (Hons.) A THESIS SUBMITTED FOR THE DEGREE OF DOCTOR OF PHILOSOPHY DEPARTMENT OF BIOLOGICAL SCIENCES THE NATIONAL UNIVERSITY OF SINGAPORE 2005 This is dedicated to my dad, mum and brothers. i ACKNOWLEDGEMENTS I am grateful to the many individuals and teams from various institutions who have contributed to the completion of this thesis in various avenues, of which encouragement was the most appreciated. They are, not in any order of preference, from the National University of Singapore (NUS): A/P Peter Ng, Tan Heok Hui, Kelvin K. P. Lim, Darren C. J. Yeo, Tan Swee Hee, Daisy Wowor, Lim Cheng Puay, Malcolm Soh, Greasi Simon, C. M. Yang, H. K. Lua, Wang Luan Keng, C. F. Lim, Yong Ann Nee; from the National Parks Board (Singapore): Lena Chan, Sharon Chan; from the Nature Society (Singapore): Subaraj Rajathurai, Andrew Tay, Vilma D’Rozario, Celine Low, David Teo, Rachel Teo, Sutari Supari, Leong Kwok Peng, Nick Baker, Tony O’Dempsey, Linda Chan; from the Wildlife Department (Malaysia): Lim Boo Liat, Sahir bin Othman; from the Forest Research Institute of Malaysia (FRIM): Norsham Yaakob, Terry Ong, Gary Lim; from WWF (Malaysia): Jeet Sukumaran; from the Economic Planning Unit, Malaysia (EPU): Puan Munirah; from the University of Sarawak (UNIMAS): Indraneil Das; from the National Science Museum, Thailand: Jairujin Nabhitabhata, Tanya Chan-ard, Yodchaiy Chuaynkern; from the University of Kyoto: Masafumi Matsui; from the University of the Ryukyus: Hidetoshi Ota; from my Indonesian friends: Frank Bambang Yuwono, Ibu Mumpuni (MZB), Djoko Iskandar (ITB); from the Philippine National Museum (PNM): Arvin C. -

Herpetological Journal SHORT NOTE
Volume 31 (January 2021), 55-59 Herpetological Journal SHORT NOTE https://doi.org/10.33256/31.1.5559 Published by the British Comparisons of image-matching software when identifyingHerpetological Society pool frog (Pelophylax lessonae) individuals from a reintroduced population Josh Dawson1, Connor T. Panter1 & Inga Zeisset1 1Ecology, Conservation and Zoonosis Research and Enterprise Group, School of Pharmacy and Biomolecular Sciences, University of Brighton, Brighton, UK Photographic identification of individual animals is a the northern-clade in Sweden (Zeisset & Hoogesteger, non-invasive and cost-effective method that can provide 2018). Globally, the species is listed as ‘Least Concern’ demographic information on wild populations. This study on the IUCN Red List of Threatened Species but has a aims to compare two photo-matching algorithms (Wild- declining population trend and a need for conservation ID and I3S-Spot) using a reintroduced population of pool intervention has been recognised (Kuzmin et al., 2009). frogs (Pelophylax lessonae) in the UK as a case study. We Pool frogs have distinctive and variable spotted skin compared the following parameters 1) sex and age, 2) patterns with a pale dorsal stripe, and exhibit some sexual image quality, 3) image collection size and 4) processing dimorphism whereby adult males have paler dorsal base- time to evaluate successful image match rates. There were colours than females (Hoogesteger et al., 2013). The no significant differences in successful match rates found presence of distinguishable morphological features and between sex and age groups. Wild-ID was more sensitive a need for adequate and minimally invasive population to image quality than I3S-Spot. There was a significant monitoring, mean that pool frogs are a suitable negative relationship between image collection size and species for non-invasive photographic monitoring successful match rates for I3S-Spot, however, no such techniques.