The Hormonal Effects of Alcohol Use on the Mother and Fetus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Puberty in Girls: Discussing Masturbation
PUBERTY IN GIRLS: DISCUSSING MASTURBATION Discussing masturbation is an anxiety-provoking moment for any parent. It is important to address the topic with your daughter in a manner that is consistent with your family’s belief system and to set rules that are both age appropriate and comfortable for you to follow through with. This includes acknowledging that it is normal for your daughter to have sexual urges and interest. A good way to open the conversation is through books that discuss puberty and sexual topics in a frank and straightforward manner. Find out what your daughter already knows. Make sure she knows the different parts of her body and their functions. Consider using picture books or a body puzzle to make a simple game such as “find the body part” to see if your daughter understands what the body parts are and their functions; give her a healthy reward or praise to show her that she has done well. Read books together about puberty/adolescence, OR if your daughter doesn’t want to read with you, make them available to her by placing them in places where she plays. When it comes to discussing masturbation, you will need to be explicit. Because many individuals on the autism spectrum tend to self-stimulate in various ways, boundaries must be set around masturbation. Teach rules for appropriate time and place, and tell your daughter that sometimes masturbation is not an option. Provide her with private time where she will be undisturbed. Establish an open dialogue with your daughter about sexuality, which includes being safe and socially appropriate. -

Original Article HISTOLOGICAL CHARACTERISTICS of FOLLICULOGENESIS in MURRAH WATER BUFFALOES DURING the EARLY POSTPUBERTAL PERIOD
Bulgarian Journal of Veterinary Medicine, 2020, 23, No 1, 8088 ISSN 1311-1477; DOI: 10.15547/bjvm.2156 Original article HISTOLOGICAL CHARACTERISTICS OF FOLLICULOGENESIS IN MURRAH WATER BUFFALOES DURING THE EARLY POSTPUBERTAL PERIOD V. MANOV, V. PLANSKI & G. S. POPOV Faculty of Veterinary Medicine, University of Forestry, Sofia, Bulgaria Summary Manov, V., V. Planski & G. S. Popov, 2020. Histological characteristics of folliculogenesis in Murrah water buffaloes during the early postpubertal period. Bulg. J. Vet. Med., 23, No 1, 8088. A characteristic feature of water buffalo heifers is that they approach breeding maturity later than bovine heifers. From a physiological and endocrinological view, this is related to a later puberty, which affects the overall reproductive performance of water buffalo. The aim of this study was to highlight some morphological characteristics of the water buffalo (Bubalus bubalis) ovaries in the early postpubertal period. The results showed active ovaries of the examined specimens. Some of the follicles had no oocyte, but were with normal structure and physiological activity. Histology is a de- finitive method for examination of ovarian activity in water buffaloes. In some of the ovulating folli- cles the oocyte was absent during early puberty. The presence of corpora lutea confirmed the endo- crine maturity of the hypothalamus-pituitary-gonadal endocrine axis in 11–14 months old heifers despite the absence of oocytes. Key words: corpus luteum, estrus, follicle, ovary, ovulation, postpubertal period, water buffalo heifer INTRODUCTION Water buffalo heifers attain breeding ma- variable and is influenced by a wide vari- turity later than bovine heifers which is ety of factors, including climate, geo- attributed to later onset of puberty, affect- graphic area, breed, season of birth, and ing the overall reproductive performance. -

Puberty—Ready Or Not Expect Some Big Changes
puberty—ready or not expect some big changes Puberty is the time in your life when your Zits! body starts changing from that of a child to that of an Girls & Boys. adult. At times you may feel like your body is totally Another change that out of control! Your arms, legs, hands, and feet happens during puberty is that your skin gets oilier and you may may grow faster than the rest of your body. You may feel a little start to sweat more. This is because your glands are growing too. clumsier than usual. It’s important to wash every day to keep your skin Compared to your friends you may feel too tall, too short, too clean. Most people use a deodorant or antiperspirant to keep odor fat, or too skinny. You may feel self-conscious about and wetness under control. Don’t be surprised, even if you wash these changes, but many of your friends probably do too. your face every day, that you still get pimples. This is called acne, and it’s normal during this time when your hormone levels are Everyone goes through puberty, but not always at high. Almost all teens get acne at one time or another. the same time or exactly in the same way. In general, here’s Whether your case is mild or severe, there are things you can do what you can expect. to keep it under control. For more information on controlling acne, talk with your pediatrician. When? There’s no “right” time for puberty to begin. -
Knowledge About Human Reproduction and Experience of Puberty 4
KNOWLEDGE ABOUT HUMAN REPRODUCTION AND EXPERIENCE OF PUBERTY 4 4.1 KNOWLEDGE AND EXPERIENCE OF PUBERTY Knowledge of the physiology of human reproduction and the means to protect oneself against sexual or reproductive problems and diseases should be available to adolescents. Better knowledge of these subjects among young adults will lead to correct attitudes and responsible reproductive health behavior. 4.1.1 Knowledge of Physical Changes In the 2002-2003 Indonesia Young Adult Reproductive Health Survey (IYARHS), respondents were asked several questions to measure their knowledge about human reproduction and the experience of puberty. They were asked to name any physical changes that a boy or a girl goes through during the transition from childhood to adolescence. The responses were spontaneous, without any prompting from the interviewer. The findings are presented in Table 4.1. It is interesting to note that while the respondents may have experienced some of the physical changes listed in the questionnaire, some may not have recognized them as part of the process of growing up into adulthood; others may not report them to the interviewer. Table 4.1 Knowledge of physical changes at puberty Percentage of unmarried women and men age 15-24 who know of specific physical changes in a boy and a girl at puberty, by age, IYARHS 2002-2003 Women Men Indicators of physical changes 15-19 20-24 Total 15-19 20-24 Total In a boy Develop muscles 26.3 27.7 26.8 33.1 30.4 32.0 Change in voice 52.2 65.6 56.7 35.5 44.6 39.2 Growth of facial hair, pubic hair, -

The Effects of Alcohol in Newborns Efeitos Do Álcool No Recém-Nascido
REVIEW The effects of alcohol in newborns Efeitos do álcool no recém-nascido Maria dos Anjos Mesquita* ABSTRACT alcoólicas leva a prejuízos individuais, para a sua família e para The purpose of this article was to present a review of the effects toda a sociedade. Apesar disso, a dificuldade do seu diagnóstico e of alcohol consumption by pregnant mothers on their newborn. a inexperiência dos profissionais de saúde faz com que o espectro Definitions, prevalence, pathophysiology, clinical features, diagnostic dessas lesões seja pouco lembrado e até desconhecido. As lesões criteria, follow-up, treatment and prevention were discussed. A causadas pela ação do álcool no concepto são totalmente prevenidas search was performed in Medline, LILACS, and SciELO databases se a gestante não consumir bebidas alcoólicas durante a gestação. using the following terms: “fetus”, “newborn”, “pregnant woman”, Assim, é fundamental a detecção das mulheres consumidoras “alcohol”, “alcoholism”, “fetal alcohol syndrome”, and “alcohol- de álcool durante a gravidez e o desenvolvimento de programas related disorders”. Portuguese and English articles published from específicos de alerta sobre as consequências do álcool durante a 2000 to 2009 were reviewed. The effects of alcohol consumed by gestação e amamentação. pregnant women on newborns are extremely serious and occur frequently; it is a major issue in Public Health worldwide. Fetal alcohol Descritores: Bebidas alcoólicas/efeitos adversos; Feto; Recém-nascido; spectrum disorders cause harm to individuals, their families, and the Síndrome alcoólica fetal; Transtornos relacionados ao uso de álcool entire society. Nevertheless, diagnostic difficulties and inexperience of healthcare professionals result in such damage, being remembered rarely or even remaining uncovered. -

Puberty in Boys: from Physical Changes to Masturbation
PUBERTY IN BOYS: FROM PHYSICAL CHANGES TO MASTURBATION Boys grow and develop (both mentally and physically) at different rates and ages. It is important to know when the “right” time is to begin talking with your son about his development. Ideally, you should begin introducing your son to his body, including his genitals, at an early age. Then, when it is time to talk about the sexual function of his body, it may not be as difficult. Use your judgment in determining when your son is ready for a conversation about puberty and sexuality. For many boys, this may be around age 9 to 11. Keep in mind that it may be earlier or later, depending on your child’s development. Whatever the age, it is important to think about where to begin. Find out what your son knows. Does he already know the body parts? Does he know what it means to have an erection? Use visuals such as drawings and pictures, or use a hand held mirror to help find out if he can name his body parts and genitals and tell you the function of each part. When talking with your son about his body, use the proper or real names of each body part, instead of just saying “down there.” Also teach your son the slang terms for male and female body parts; he is likely to hear them at school or elsewhere. Keep it SIMPLE. For example: “This is your penis: this is where the urine/pee comes out when you use the toilet.” “This is your anus: this is where the stool/poop comes out after your food has been digested.” “This is your penis: this is where semen comes out when you ejaculate.” Be POSITIVE and tell him that his body will grow taller, his testicles and penis will grow bigger, and hair will grow under his arms and in his groin area and that it is NORMAL. -

Female Tanner Stages (Sexual Maturity Rating)
Strength of Recommendations Preventive Care Visits – 6 to 17 years Bold = Good Greig Health Record Update 2016 Italics = Fair Plain Text = consensus or Selected Guidelines and Resources – Page 3 inconclusive evidence The CRAFFT Screening Interview Begin: “I’m going to ask you a few questions that I ask all my patients. Please be honest. I will keep your Screening for Major Depressive Disorder -USPSTF answers confidential.” Age 12 years to 18 years 7 to 11 yrs No Yes Part A During the past 12 months did you: Screen (when systems in place for diagnosis, treatment and Insufficient 1. Drink any alcohol (more than a few sips)? □ □ follow-up) evidence 2. Smoked any marijuana or hashish? □ □ Risk factors- parental depression, co-morbid mental health or chronic medical 3. Used anything else to get high? (“anything else” includes illegal conditions, having experienced a major negative life event drugs, over the counter and prescription drugs and things that you sniff or “huff”) □ □ Tools-Patient Health Questionnaire for Adolescent(PHQ9-A) Tools For clinic use only: Did the patient answer “yes” to any questions in Part A? &Beck Depression Inventory-Primary Care version (BDI-PC) perform less No □ Yes □ well Ask CAR question only, then stop. Ask all 6 CRAFFT questions Treatment-Pharmacotherapy – fluoxetine (a SSRI) is Part B Have you ever ridden in a CAR driven by someone □ □ efficacious but SSRIs have a risk of suicidality – consider only (including yourself) who was ‘‘high’’ or had been using if clinical monitoring is possible. Psychotherapy alone or alcohol or drugs? combined with pharmacotherapy can be efficacious. -

Precocious Puberty Children with Spina Bi�Da and Hydrocephalus May Start Puberty Earlier Than Their Peers
SBA National Resource Center: 800-621-3141 Precocious Puberty Children with Spina Bida and hydrocephalus may start puberty earlier than their peers. What is Puberty? If major breast development starts before age 8, it is considered early. (Sometimes girls will have some Puberty refers to normal body changes that lead to breast development, with no other signs of puberty. maturity and the ability to have children. Normal puberty This isolated change may be normal.) begins between ages 8 and 12 in girls and between 9 and 14 in boys. Hormones made in the brain control the timing and sequence of puberty. These hormones What are the stages of normal puberty in boys? stimulate other parts of the body to make sex hormones. The usual sequence in boys is: The sex hormones, especially estrogen in girls and testosterone in boys, cause sexual maturation. • The testicles grow larger. • The penis grows larger. What are the stages of normal puberty in girls? • Pubic hair grows. The physical changes seen in puberty are labeled by “Tanner staging.” Stage 1 is child-like (before puberty) • There is a growth spurt.rt. and stage 5 is full maturity. The usual sequence in girls is: • Other body hair grows.s. • Breasts start to develop. If boys show major developmentelopment • Hips widen and a there is a growth spurt that usually before age 9, it is considereddered lasts about three to four years. early. Early puberty in girls or boys is called • Pubic hair grows (three-to-six months after breasts “Precocious Puberty.” develop). • Other body hair grows. -

FASD Effects of Alcohol on a Fetus
EFFECTS OF ALCOHOL ON A FETUS “Of all the substances of abuse (including cocaine, heroin, and marijuana), alcohol produces by far the most serious neurobehavioral effects in the fetus.” —Institute of Medicine Report to Congress, 19961 Prenatal exposure to alcohol can damage a fetus at any time, causing problems that persist throughout the individual’s life. There is no known safe level of alcohol use in pregnancy. WHAT IS THE SCOPE OF THE PROBLEM? identified in virtually every part of the body, including the brain, face, eyes, ears, heart, kidneys, and bones. No Alcohol is one of the most dangerous teratogens, which single mechanism can account for all the problems that are substances that can damage a developing fetus.1 Every alcohol causes. Rather, alcohol sets in motion many time a pregnant woman has a drink, her unborn child processes at different sites in the developing fetus: has one, too. Alcohol, like carbon monoxide from cigarettes, passes easily through the placenta from the • Alcohol can trigger cell death in a number of ways, mother's bloodstream into her baby's blood (See Figure causing different parts of the fetus to develop abnormally. 1)—and puts her fetus at risk of having a fetal alcohol • Alcohol can disrupt the way nerve cells develop, travel spectrum disorder (FASD). The blood alcohol level to form different parts of the brain, and function. (BAC) of the fetus becomes equal to or greater than the blood alcohol level of the mother. Because the fetus • By constricting the blood vessels, alcohol interferes with cannot break down alcohol the way an adult can, its BAC blood flow in the placenta, which hinders the delivery 2 remains high for a longer period of time. -

Post-Orgasmic Illness Syndrome: a Closer Look
Indonesian Andrology and Biomedical Journal Vol. 1 No. 2 December 2020 Post-orgasmic Illness Syndrome: A Closer Look William1,2, Cennikon Pakpahan2,3, Raditya Ibrahim2 1 Department of Medical Biology, Faculty of Medicine and Health Sciences, Universitas Katolik Indonesia Atma Jaya, Jakarta, Indonesia 2 Andrology Specialist Program, Department of Medical Biology, Faculty of Medicine, Universitas Airlangga – Dr. Soetomo Hospital, Surabaya, Indonesia 3 Ferina Hospital – Center for Reproductive Medicine, Surabaya, Indonesia Received date: Sep 19, 2020; Revised date: Oct 6, 2020; Accepted date: Oct 7, 2020 ABSTRACT Background: Post-orgasmic illness syndrome (POIS) is a rare condition in which someone experiences flu- like symptoms, such as feverish, myalgia, fatigue, irritabilty and/or allergic manifestation after having an orgasm. POIS can occur either after intercourse or masturbation, starting seconds to hours after having an orgasm, and can be lasted to 2 - 7 days. The prevalence and incidence of POIS itself are not certainly known. Reviews: Waldinger and colleagues were the first to report cases of POIS and later in establishing the diagnosis, they proposed 5 preliminary diagnostic criteria, also known as Waldinger's Preliminary Diagnostic Criteria (WPDC). Symptoms can vary from somatic to psychological complaints. The mechanism underlying this disease are not clear. Immune modulated mechanism is one of the hypothesis that is widely believed to be the cause of this syndrome apart from opioid withdrawal and disordered cytokine or neuroendocrine responses. POIS treatment is also not standardized. Treatments includeintra lymphatic hyposensitization of autologous semen, non-steroid anti-inflamation drugs (NSAIDs), steroids such as Prednisone, antihistamines, benzodiazepines, hormones (hCG and Testosterone), alpha-blockers, and other adjuvant medications. -

Trends and Patterns in Menarche in the United States: 1995 Through 2013–2017 by Gladys M
National Health Statistics Reports Number 146 September 10, 2020 Trends and Patterns in Menarche in the United States: 1995 through 2013–2017 By Gladys M. Martinez, Ph.D. Abstract older, have older friends, and be more likely to engage in negative behaviors Objective—This report presents national estimates of age at first menstrual period such as missing school, smoking, and for women aged 15–44 in the United States in 2013–2017 based on data from the drinking (8–11). The younger the age at National Survey of Family Growth (NSFG). Estimates for 2013–2017 are compared first menstrual period and first sexual with those from previous NSFG survey periods (1995, 2002, and 2006–2010). intercourse, the longer the interval Methods—Data for all survey periods analyzed are based on in-person interviews young women will potentially spend at with nationally representative samples of women in the household population aged risk of pregnancy. Differences in age at 15–44 in the United States. For the 2013–2017 survey period, interviews were menarche across population subgroups conducted with 10,590 female respondents aged 15–44. In 2015–2017, the age range may help explain differences in timing of the NSFG included women aged 15–49, but only those aged 15–44 were included of first sexual intercourse and timing of in this analysis. The response rate for the 2013–2017 NSFG was 67.4% for women. first births. The relationship between age Measures of menarche in this report include average age at first menstrual period, at menarche and the timing of first sexual probability of first menstrual period at each age, and the relationship between age at intercourse in the United States has menarche and age at first sexual intercourse. -
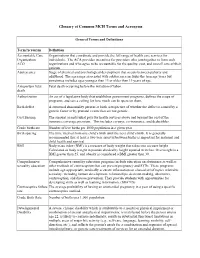
Glossary of Common MCH Terms and Acronyms
Glossary of Common MCH Terms and Acronyms General Terms and Definitions Term/Acronym Definition Accountable Care Organizations that coordinate and provide the full range of health care services for Organization individuals. The ACA provides incentives for providers who join together to form such ACO organizations and who agree to be accountable for the quality, cost, and overall care of their patients. Adolescence Stage of physical and psychological development that occurs between puberty and adulthood. The age range associated with adolescence includes the teen age years but sometimes includes ages younger than 13 or older than 19 years of age. Antepartum fetal Fetal death occurring before the initiation of labor. death Authorization An act of a legislative body that establishes government programs, defines the scope of programs, and sets a ceiling for how much can be spent on them. Birth defect A structural abnormality present at birth, irrespective of whether the defect is caused by a genetic factor or by prenatal events that are not genetic. Cost Sharing The amount an individual pays for health services above and beyond the cost of the insurance coverage premium. This includes co-pays, co-insurance, and deductibles. Crude birth rate Number of live births per 1000 population in a given year. Birth spacing The time interval from one child’s birth until the next child’s birth. It is generally recommended that at least a two-year interval between births is important for maternal and child health and survival. BMI Body mass index (BMI) is a measure of body weight that takes into account height.