Alcohol Withdrawal and Intoxication Guideline
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Alcohol Intoxication & Intervention Scale
Alcohol Intoxication & Intervention Scale Alcohol affects each individual differently. The effect of alcohol on a person will vary according to the person's mood, the time of day, Standard Drink amount of food in the stomach, the mixer used, how fast the person One standard drink of beer drinks, and what and why they are drinking. There are a variety of One 12 oz bottle of beer positive and negative consequences related to drinking. One 12 oz can of beer One 8 oz glass of malt liquor (i.e. Blood Alcohol Level Old English, Mickey's) Once you know the definition of one standard drink (see chart to the One standard drink of wine right), you can estimate your Blood Alcohol Level (BAL). BAL levels One 4 oz glass of wine (pictured) represent the percent of your blood that is concentrated with alcohol. A One 3 ‐ 3.5 oz of fortified wine (i.e. BAL of .10 means that .1% of your bloodstream is composed of alcohol. port, sherry) One bottle of table wine is about 5 Some key factors that affect BAL: standard drinks How many standard drinks you drink One standard drink of hard alcohol Remember different drinks have different strengths either One 1.25 oz shot of hard liquor because of differences in proofs of hard liquor or because some (pictured) drinks contain more than one shot One mixed drink containing one 1.25 oz shot of hard liquor Food eaten along with drinking alcohol will result in a lower, One 750ml bottle of hard liquor ("a delayed BAL because the alcohol enters the bloodstream at a fifth") is about 17 standard drinks lower rate Intoxication and Intervention Scale Know the visible signs of intoxication. -

Alcohol Intoxication Withdrawal Adult
Provincial Clinical Knowledge Topic Alcohol Intoxication Withdrawal, Adult Emergency Department V 1.5 © 2018, Alberta Health Services. This work is licensed under the Creative Commons Attribution-Non-Commercial-No Derivatives 4.0 International License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/. Disclaimer: This material is intended for use by clinicians only and is provided on an "as is", "where is" basis. Although reasonable efforts were made to confirm the accuracy of the information, Alberta Health Services does not make any representation or warranty, express, implied or statutory, as to the accuracy, reliability, completeness, applicability or fitness for a particular purpose of such information. This material is not a substitute for the advice of a qualified health professional. Alberta Health Services expressly disclaims all liability for the use of these materials, and for any claims, actions, demands or suits arising from such use. Document History Version Date Description of Revision Completed By / Revised By 1.1 July 2015 Completed document (2013) reformatted into Dr. Bullard / Carla new topic template Milligan 1.2 January Minor edits in the Rationale section and form 1 Dr. Bullard / Sarah 2016 info in general care section as well as addition Searle of CIWA-Ar Scoring Reference tool to appendix 1.3 May 2016 Minor edits made to working group Sarah Searle membership list 1.4 June Removed link to Center for Addiction and Dr. Bullard / Sarah 2017 Mental Health assessment and documentation Searle form on pg. 35. Documentation requirements will continue as per local practice at this time. -

Mechanisms of Ethanol-Induced Cerebellar Ataxia: Underpinnings of Neuronal Death in the Cerebellum
International Journal of Environmental Research and Public Health Review Mechanisms of Ethanol-Induced Cerebellar Ataxia: Underpinnings of Neuronal Death in the Cerebellum Hiroshi Mitoma 1,* , Mario Manto 2,3 and Aasef G. Shaikh 4 1 Medical Education Promotion Center, Tokyo Medical University, Tokyo 160-0023, Japan 2 Unité des Ataxies Cérébelleuses, Service de Neurologie, CHU-Charleroi, 6000 Charleroi, Belgium; [email protected] 3 Service des Neurosciences, University of Mons, 7000 Mons, Belgium 4 Louis Stokes Cleveland VA Medical Center, University Hospitals Cleveland Medical Center, Cleveland, OH 44022, USA; [email protected] * Correspondence: [email protected] Abstract: Ethanol consumption remains a major concern at a world scale in terms of transient or irreversible neurological consequences, with motor, cognitive, or social consequences. Cerebellum is particularly vulnerable to ethanol, both during development and at the adult stage. In adults, chronic alcoholism elicits, in particular, cerebellar vermis atrophy, the anterior lobe of the cerebellum being highly vulnerable. Alcohol-dependent patients develop gait ataxia and lower limb postural tremor. Prenatal exposure to ethanol causes fetal alcohol spectrum disorder (FASD), characterized by permanent congenital disabilities in both motor and cognitive domains, including deficits in general intelligence, attention, executive function, language, memory, visual perception, and commu- nication/social skills. Children with FASD show volume deficits in the anterior lobules related to sensorimotor functions (Lobules I, II, IV, V, and VI), and lobules related to cognitive functions (Crus II and Lobule VIIB). Various mechanisms underlie ethanol-induced cell death, with oxidative stress and Citation: Mitoma, H.; Manto, M.; Shaikh, A.G. Mechanisms of endoplasmic reticulum (ER) stress being the main pro-apoptotic mechanisms in alcohol abuse and Ethanol-Induced Cerebellar Ataxia: FASD. -

Court Intervention: Pre-Sentence Investigation
If you have issues viewing or accessing this file contact us at NCJRS.gov. ------~--- ---- .. National Criminal Justice Reference Service COURT INTERVENTION: PRE-SENTENCE This microfiche was 'produced from documents received for inclusion in the NCJRS data base. Since NCJRS cannot exercise control over the physical condition of the documents submitted, INVESTIGATION the individual frame quality will vary. The resolution chart on this frame may be used to evaluate the document quality. TECHNIQUES FOR DRINKING/DRIVING OFFENSES 2 8 1.0 :; 11111 . 111111:~ W IIp·2 .2 W I.r.: I~ w: J:,i 14.0 ..,.I.::. u. - 1.1 tIl&.:.u - , == I 111111.25 11111·1.4 111111.6 I ! I MICROCOPY RESOLUTION TEST CHART NATIONAL BUREAU OF STANDARDS-1963-A .. ' ~ ...,.. '~'''';'''' ..' .. ~" - q P ARTI'CIPANT'S Microfil~ing proced~;~~~sed to create this fiche comply with & the standards set forth i1141CFR lOl-11.SD4. MANUAL Points of view or opinions stated in this document are those of the author(s) and do not represent the official U.S. Department of Transportation DATE FILMED ~ National Highway Traffic Safety Administration position or policies of the U. S. Department of Justice. Washington, D.C. 20590 '-, . ~ , .... ~ 1. _ .I. ' 1 9/04/81' " National Institute of Justice -;: .J.____ .... United States Department of Justice Washington, D. C. 2053<1 • , --~---.~--~. --- ~ I ! CONTENTS SEMINAR AGENDA iii ; - l AGENDA PRE-SENTENCE INVESTIGATION SEMINAR Day One 0900-1200 1. Introduction and Overview UNITS 1. Introduction and Overview 1 i: This unit covers: (a) introduction and administrative infor 2. The Problem Drinking Drive 9 mation, (b) information on DOT/NHTSA standards, (c) the genesis of the project, and (d) explanation of the ASAP 3. -

Physician's Newsletter
PUBLISHED· BY THE N. Y. C. MEDICAL SOCIETY PHYSICIAN'S ON ALCOHOLISM, Inc. 167 East 80 St. New York 21, N.Y. A MARCH 1966 VOL. I, NO.2 ©Copyright 1966 The N.Y.C. Medical. NEWSLETTER Society on Alcoholism, Inc. I -HISTORIC RULING I 'TOTAL ABSTINENCE' TERMED A U.S. Court of Appeals tribunal in OBJECTIVE OF TREATMENT Richmond, Va., has rulee unanimously Alcoholic patients whose treatment led to total abstinence had the best chance that it is "cruel and unusual punish of marked improvement of social adaptation, according to Dr. D. L. Davies, director ment" to arrest and treat a chronic alco of psychiatry at Maudsley Hospital, London, England, a major teaching center. He holic as a criminal; he might, however, spoke at a seminar at Norwalk Hospital, Norwalk, Conn., about an. article .of. :I.Jis · be held for medical treatment. which had been widely thought to endorse treatment directed at·a return to so6Ial The case involved an individual who drinking. had been convicted of public intoxica Misunderstandings had arisen as a result of his report, "Normal Drinking tion more than 200 times and who esti in Reversed Alcoholics" published last mates that he has spent more than two year in the Quarterly Journal of Alcohol thirds of his life in jail. Studies. The article reports the elabo CENTRAL ISLIP OPENS Crux of the decision, which set aside rate follow-up studies of patients treated ALCOHOL FACILITY a 2-year sentence, was the court's state for alcoholism in Maudsley Hospital, in Dedication of the Charles K. -

Background Paper 6.14 Harmful Use of Alcohol Alcohol Use Disorders and Alcoholic Liver Diseases
Priority Medicines for Europe and the World "A Public Health Approach to Innovation" Update on 2004 Background Paper Written by Warren Kaplan, Ph.D, JD, MPH Background Paper 6.14 Harmful use of Alcohol Alcohol Use Disorders and Alcoholic Liver Diseases By Rene Soria Saucedo (MD, MPH, PhDc) January 2013 Update on 2004 Background Paper, BP 6.14 Alcohol Use Disorders Table of Contents Abbreviations: ..................................................................................................................................................... 5 Executive Summary ............................................................................................................................................ 6 Burden of Disease ............................................................................................................................................ 6 Treatment Options ........................................................................................................................................... 7 1. Introduction ................................................................................................................................................. 8 1.1 Alcohol Consumption (ACo) and its relationship with Alcohol Use Disorders (AUD) ........... 8 1.2 Alcohol Use Disorders (AUD) .......................................................................................................... 9 1.3 Alcoholic Liver Diseases (ALD) ..................................................................................................... -

Reducing the Negative Consequences of Drinking and Alcohol Intoxication
European status report on alcohol and health 2014 Reducing the negative consequences of drinking and alcohol intoxication Reducing the negative consequences of drinking and alcohol intoxication Background The pattern of drinking is just as important for alcohol-related harm as the volume of alcohol consumed. Most alcohol is consumed in heavy drinking occasions, which is the most risky pattern of drinking and one that can cause harm not only to the drinkers themselves but also to others. Taking action in drinking environments is one important strategy for reducing the frequency and size of heavy drinking patterns.1 Pubs, bars and nightclubs are key locations for the consumption of alcohol, particularly among young people. These drinking venues can be both a hub of social and recreational activity and a source of revenue for local economies. However, the congregation of large numbers of drinkers in drinking environments means they are often associated with high levels of intoxication and alcohol-related harm, including violence, road traffic crashes, public disorder and unintentional injury. Factors that have been associated with problematic venues include a permissive atmosphere, crowding, low levels of comfort, poorly trained staff and cheap drinks promotions.2 Interventions in drinking environments can reduce alcohol-related harm by targeting both the way in which alcohol is served and the conditions in which it is consumed, resulting in wide- ranging benefits that include averting problems that often harm people who are not drinking, -

Blind Drunk: Alcoholic Pancreatitis and Loss Ofvision James R
CLINICAL REPORTS 151 Postgrad Med J: first published as 10.1136/pgmj.69.808.151 on 1 February 1993. Downloaded from Postgrad Med J (1993) 69, 151 - 152 A) The Fellowship of Postgraduate Medicine, 1993 Blind drunk: alcoholic pancreatitis and loss ofvision James R. Steel, John R. Cockcroft and James M. Ritter Department ofClinical Pharmacology, Guy's Hospital Medical School, London SE] 9RT, UK Summary: Acute loss of vision and alcoholic intoxication suggests the possibility of methanol poisoning. In this report we describe an alcoholic patient who complained of blindness after recovering from alcoholic pancreatitis and delirium tremens. Visual acuity was severely impaired and fundoscopy revealed florid bilateral cotton wool spots and a cherry red spot at the right macula. Such appearances have previously been reported in cases of post-traumatic visual loss, and may share a common aetiology of fat embolization. The association between alcoholic pancreatitis and visual loss is rare, and fundal examination should be performed on all patients with pancreatitis, especially those who complain ofvisual disturbance. Introduction Previous case reports have confirmed an associa- agitated state. Blood ethanol was 63.8 mg/l00 ml, tion between severe alcoholic pancreatitis and 'y-glutamyl transpeptidase was raised at 206 U/l visual loss.'"2 These changes are remarkably similar (normal range 11- 54), aspartate transaminase to those seen in post-traumatic retinopathy. raised at 46 U/l (normal range < 23) and amylase Protected by copyright. Although blindness associated with alcohol abuse raised at 809 U/l (normal range < 220). There was suggests the possibility of methanol ingestion, it is a neutrophil leucocytosis with total white cell count important to consider the possibility of a retino- of 17.1 x 109/l and the mean corpuscular volume pathy due to alcoholic pancreatitis and fundal was 102 fl (normal range 76-96). -

Acute Alcohol Intoxication May Cause Delay in Stroke Treatment
Arokszallasi et al. BMC Neurology (2019) 19:14 https://doi.org/10.1186/s12883-019-1241-6 CASEREPORT Open Access Acute alcohol intoxication may cause delay in stroke treatment – case reports Tamas Arokszallasi1*, Eszter Balogh1, Laszlo Csiba1,2, Istvan Fekete1, Klara Fekete1 and Laszlo Olah1 Abstract Background: The signs and symptoms of acute alcohol intoxication resemble those of vertebrobasilar stroke. Due to their shared symptoms including double vision, nystagmus, dysarthria, and ataxia, the differential diagnosis of alcohol intoxication and vertebrobasilar stroke may pose a challenge. Moreover, if alcohol intoxication and stroke occur simultaneously, the signs and symptoms of stroke may be attributed to the effects of alcohol, leading to delayed stroke diagnosis and failure to perform reperfusion therapy. Case presentations: Three cases of alcohol intoxication and stroke are presented. The first patient (female, 50 years old) had dysarthria, nystagmus and trunk ataxia on admission. Her blood alcohol level was 2.3‰. The symptoms improved after forced diuresis, but 5.5 h later progression was observed, and the patient developed diplopia and dysphagia in addition to her initial symptoms. Angiography showed occlusion of the basilar artery. Intraarterial thrombolysis was performed. The second patient (male, 62 years old) developed diplopia, dysarthria and trunk ataxia after consuming 4-units of alcohol, and his symptoms were attributed to alcohol intoxication. Two hours later, neurological examination revealed dysphagia and mild right-sided hemiparesis, which questioned the causal relationship between the symptoms and alcohol consumption. Cerebral CT was negative, and intravenous thrombolysis was administered. The third patient (male, 55 years old) consumed 10 units of alcohol before falling asleep. -

The Effect of Alcohol Consumption on Risk for Sepsis and ARDS
The Effect of Alcohol Consumption on Risk for Sepsis and ARDS E. L. Burnham, M. Moss, and G. S. Martin I Introduction Alcohol is the most frequently abused drug throughout the world, and alcohol-re lated problems are a common occurrence among patients admitted to hospitals and intensive care units (ICUs). Alcohol affects all tissues of the body. Its effects on im mune function and the systemic inflammatory response syndrome (SIRS) remain topics of active investigation. In regard to immune function, alcohol consumption alters the response at several points along the inflammatory cascade. Due to these potent modulating effects on immune function, alcoholic patients have an increased incidence and severity of infection, particularly in the lung. This association be tween alcohol use and infection is especially evident in the post-operative setting. Among patients with sepsis, a prior history of chronic alcohol abuse confers a sig nificant increase in the likelihood of respiratory dysfunction and development of acute respiratory distress syndrome (ARDS). Chronic alcohol abuse similarly in creases the mortality from ARDS. One possible mechanism by which chronic alco hol abuse may increase susceptibility to acute lung injury (ALI} is through altera tions in pulmonary glutathione homeostasis. This chapter discusses the immuno modulatory effects of alcohol and the epidemiological and experimental evidence associating chronic alcohol abuse, sepsis, and the development of ARDS. I Background Alcohol is the most commonly abused drug in the world, with approximately half the US population consuming alcohol regularly. Fifteen to twenty million individuals meet the criteria for alcoholism [ 1]. The economic cost of alcohol in the USA is around $100 billion (USD), with > 10% of this cost directly attributable to medical services [2]. -
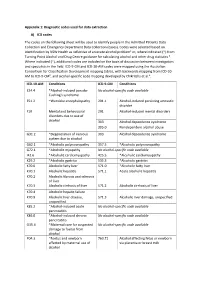
Diagnostic Codes Used for Data Extraction A) ICD Codes the Codes on the Following Sheet Will Be Used to Identify
Appendix 1: Diagnostic codes used for data extraction A) ICD codes The codes on the following sheet will be used to identify people in the Admitted Patients Data Collection and Emergency Department Data Collection (cases). Codes were selected based on identification by NSW Health as reflective of an acute alcohol problem1 or, where indicated (*) from Turning Point Alcohol and Drug Centre guidance for calculating alcohol and other drug statistics 2. Where indicated (~), additional codes are included on the basis of discussion between investigators and specialists in the field. ICD-9-CM and ICD-10-AM codes were mapped using the Australian Consortium for Classification Development mapping tables, with backwards mapping from ICD-10- AM to ICD-9-CM3, and alcohol-specific code mapping developed by Chikritzhs et al.4. ICD-10-AM Conditions ICD-9-CM Conditions E24.4 *Alcohol-induced pseudo- No alcohol-specific code available Cushing's syndrome E51.2 ~Wernicke encephalopathy 291.1 Alcohol-induced persisting amnestic disorder F10 Mental and behavioural 291 Alcohol-induced mental disorders disorders due to use of alcohol 303 Alcohol dependence syndrome 305.0 Nondependent alcohol abuse G31.2 *Degeneration of nervous 303 Alcohol dependence syndrome system due to alcohol G62.1 *Alcoholic polyneuropathy 357.5 *Alcoholic polyneuropathy G72.1 *Alcoholic myopathy No alcohol-specific code available I42.6 *Alcoholic cardiomyopathy 425.5 *Alcoholic cardiomyopathy K29.2 *Alcoholic gastritis 535.3 *Alcoholic gastritis K70.0 Alcoholic fatty liver 571.0 *Alcoholic -

Delirium Tremens
DELIRIUM TREMENS Alcohol and Narcotic Withdrawals Delirium Tremens (commonly referred to as "The Horrors", "Jazz Hands", or "The Shakes") is a psychological and physiological reaction to the sudden disruption of habitual/prolonged daily alcohol or narcotic consumption. Delirium Tremens (aka. "DT's") is also referred to as "Alcoholic Psychosis". BACKGROUND Delirium Tremens is the most severe form of alcohol withdrawal, which may result in hallucinations, seizures, and cardiovascular collapse. According to http://emedicine.medscape.com, the syndrome was first described by Thomas Sutton in 1813, but the link to alcohol abstinence wasn't made until the 1950's. Historically, older white males had a higher risk of developing severe alcohol withdrawal. Throughout the years, alcohol and drug withdrawal have been seen frequently in the corrections setting. This is due to many offenders being addicted to alcohol or drugs, and a sudden interruption of alcohol or drug use occurs when offenders are taken into custody. Because of the volatile situation of an individual withdrawing from alcohol or drugs, correctional health care providers and custodial staff should be properly trained to recognize the symptoms and ·.·. 1--r-1OOr::'.\t,1-\/cc.;•:c•;·•;L;;;;;.;;;;c;;o•;;;;;c=c•'cefCf"'''-••;•~•·cc-c_••--•'CCCc;_;'-'.; t" . ___ ,··.; .. ; res P0 nd Pro 1 ALCOHOL-RELATED DEATHS: THENAND NOW According to the Center for Disease Control (CDC), in the United States, excessive alcohol consumption was the third leading preventable cause of death in 2001. It was estimated that in 2001, approximately 75,766 deaths were attributed to the harmful effects of excessive alcohol use. In 2008, a more recent study was done, that determined that in 2001-2005, approximately 79,000 occurred annually that were attributed to excessive alcohol use.