Environmental Limitations of Two Rare Atlantic Coastal
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tusket River & Basin
Tusket River & Basin Introduction The Tusket River presents an area of 3000 square kilometres (1158 square miles) on the southwestern tip of Nova Scotia. The area consists of a coastal basin with an inland watershed associated with the great Tusket River. This name is derived from a Mi'kmaq word "Neketaouksit" meaning "Great Forked Tidal River". This unique and diverse environment has offered natural resources to the Mi'kmaq First Nation's people for 7000 years and to the French Acadian settlers since the early 17th century. In the context of sustainable development, the present-day Acadians can offer a bilingual ecotourism package of nature-adventure and discovery blended with local history and heritage. The following natural history of the area will be presented in two segments. The first section will describe the natural environment of the Tusket Basin and its interrelationships with the Acadian people. The second segment will describe the natural history of the Tusket River in a similar manner to present the Acadian in his natural environment. Basin description 1. Physical features The Tusket Basin has a width of 32 kilometres (20 miles) between headlands at Chebogue Point and Lower East Pubnico. The mainland coastline between these two boundaries is highly indented and irregular with a measure of 500 kilometres (310 miles). Elongated points, peninsulas, ridges, drumlins (low hills) and eskers are oriented North-South and are separated by many tidal channels, inlets, estuaries and bays. High tide in the estuary of the "great forked tidal river" (Tusket) carries salt water inland for 24 kilometres (15 miles). -

Atlantic Maritime Ecozone+: Evidence for Key Findings
Atlantic Maritime Ecozone+ evidence for key findings summary Canadian Biodiversity: Ecosystem Status and Trends 2010 Evidence for Key Findings Summary Report No. 3 Published by the Canadian Councils of Resource Ministers Library and Archives Canada Cataloguing in Publication Atlantic Maritime Ecozone+ evidence for key findings summary. Issued also in French under title: Sommaire des éléments probants relativement aux constatations clés pour l’écozone+ maritime de l’Atlantique. Electronic monograph in PDF format. En14-43/0-3-2015E-PDF 978-1-100-23826-5 Information contained in this publication or product may be reproduced, in part or in whole, and by any means, for personal or public non-commercial purposes, without charge or further permission, unless otherwise specified. You are asked to: Exercise due diligence in ensuring the accuracy of the materials reproduced; Indicate both the complete title of the materials reproduced, as well as the author organization; and Indicate that the reproduction is a copy of an official work that is published by the Government of Canada and that the reproduction has not been produced in affiliation with or with the endorsement of the Government of Canada. Commercial reproduction and distribution is prohibited except with written permission from the author. For more information, please contact Environment Canada’s Inquiry Centre at 1- 800-668-6767 (in Canada only) or 819-997-2800 or email to [email protected]. Cover photos: Margaree Valley, Cape Breton Island, Nova Scotia, © iStock.com / cworthy; Hopewell Rocks, Bay of Fundy, New Brunswick, © iStock.com / MorganLeFaye This report should be cited as: ESTR Secretariat. 2014. -

Overview of the Biological Characteristics of Alewife (Alosa Pseudoharengus)
Overview of the Biological Characteristics of Alewife (Alosa pseudoharengus) spawning runs on the Isthmus of Chignecto, New Brunswick, Canada. By Rachelle Breau A Thesis Submitted to Saint Mary’s University, Halifax, Nova Scotia in Partial Fulfillment of the Requirements for the Degree of Bachelor of Science. Department of Environmental Science Saint Mary’s University Halifax, Nova Scotia, Canada April 2020, Halifax, Nova Scotia © R. Breau, 2020 Approved: Dr. Laura Weir [Honours Supervisor] Approved: Dr. Linda Campbell [Reader] Date: April 9th, 2020 Overview of the Biological Characteristics of Alewife (Alosa pseudoharengus) spawning runs on the Isthmus of Chignecto, New Brunswick, Canada. By Rachelle Breau Abstract Alewife (Alosa pseudoharengus) are an anadromous species of river herring that are found in the Atlantic Ocean, ranging from the coasts of North Carolina, USA to Newfoundland, Canada. Age-structured population models and other aspects of populations have not been determined for all Alewife populations in the Maritime Provinces. To inform population models, background information are examined including biological characteristics such as age, growth patterns and condition factor. Alewives were aged by enumerating annuli on scales. Alewife weight, fork length, total length and sex were collected in the field as a part of a preexisting project from 2013 to 2019 the Inner Bay of Fundy region. Here, the presence of spatial-temporal differences in fish size, condition factor, sex ratio or ages and potential changes or biases across river systems are identified. The results indicate that length-weight relationships and growth rates did trend upward between 2013 to 2019, with female Alewife growing at a faster rate than male Alewife. -

Plymouth Gentian,Sabatia Kennedyana
COSEWIC Assessment and Status Report on the Plymouth Gentian Sabatia kennedyana in Canada ENDANGERED 2012 COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC. 2012. COSEWIC assessment and status report on the Plymouth Gentian Sabatia kennedyana in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xi + 46 pp. (www.registrelep-sararegistry.gc.ca/default_e.cfm). Previous report(s): COSEWIC. 2000. COSEWIC assessment and update status report on the Plymouth gentian Sabatia kennedyana in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vii + 10 pp. Newell, R.E. 1999. Update COSEWIC status report on the Plymouth gentian Sabatia kennedyana in Canada, in COSEWIC assessment and update status report on the Plymouth gentian Sabatia kennedyana in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 1-10 pp. Keddy, C., and P. Keddy. 1984. COSEWIC status report on the Plymouth gentian Sabatia kennedyana in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 27 pp. Production note: COSEWIC would like to acknowledge Sean Blaney and Nicholas Hill for writing the status report on Plymouth Gentian, Sabatia kennedyana, in Canada, prepared under contract with Environment Canada. This report was overseen and edited by Bruce Bennett, Co-chair of the COSEWIC Vascular Plants Specialist Subcommittee. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: 819-953-3215 Fax: 819-994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Également disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur la Sabatie de Kennedy (Sabatia kennedyana) au Canada. -

Annual Report 1961
MTMIM1 DEPARTMENT OF FISHERIES CANADA Linn 4 240 0171 FLOOR OTTAWA, ONTARIO, WEST. K IA 0E6 CANADA Being the Ninety-fifth Annual Fisheries Report of the Government of Canada 61925-4---1 ROGER DUFIAMEL, F.R.S.C. QUEEN'S PRINTER AND CONTROLLER OF STATIONERY OTTAWA, DM Price: 50 cents Cat. No: Fa 1-1961 To His Excellency Major-General Georges P. Vanier, D.S.O., C.D., Governor General and Commander-in-Chief of Canada May it Please Your Excellency: I have the honour herewith, for the information of Your Excellency and the Parliament of Canada, to present the Annual Report of the Department of Fisheries for the year 1961, and the financial statements of the Department for the fiscal year 1961-1962 Respectfully submitted, Minister of Fisheries. 61925-4- Li- To the Honourable J. A.ngus MacLean, M.P., Minister of Fisheries, Ottawa, Canada. Sir: I submit herewith the Annual Report of the Department of Fisheries for the year 1961, and the financial statements of the Department for the fiscal year 1961-1962. I have the honour to be, Sir, Your obedient servant e,6) Deputy Minister. CONTENTS PAGE Introduction 7 Conservation and Development Service 9 Departmental Vessels 32 Inspection Service 35 Economics Service 43 Information and Consumer Service 49 Industrial Development Service 56 Fishermen's Indemnity Plan 61 Fisheries Prices Support Board 63 Fisheries Research Board of Canada 65 International Commissions 80 Special Committees 99 The Fishing Industry 102 Statistics of the Fisheries 107 APPENDICES 1. Financial Statements, 1961-1962 2. Fish Culture Development Statements XXI 5 Strong arms and a stout back are requisites for a successful fisherman. -

~Nalla~R~C (CANADA a GEOLOGY FIELD "GUIDE to SELECTED SITES in NEWFOUNDLAND, NOVA SCOTIA
D~s)COVER~NGROCK~~ ~j!NERAl~ ~NfO)FOs)S~l5) ~NAllA~r~C (CANADA A GEOLOGY FIELD "GUIDE TO SELECTED SITES IN NEWFOUNDLAND, NOVA SCOTIA, PRINCE EDV\JARDISLAND7 AND NEW BRUNSWICK 7_".-- ~ _. ...._ .•-- ~.- Peter Wallace. Editor Atlantic Geoscience Society Department of Earth Sciences La Societe G60scientifique Dalhousie University de L'Atlantique Halifax, Nova Scotia AGS Special Publication 14 • DISCOVERING ROCKS, MINERALS AND FOSSILS IN ATLANTIC CANADA A Geology Field Guide to Selected Sites in Newfoundland, Nova Scotia, Prince Edward Island and New Brunswick • Peter Wallace, editor Department of Earth Sciences Dalhousie University, Halifax, Nova Scotia Atlantic Geoscience Society La Societe Geoscientifique de L'Atlantique • AGS Special Publication • @ 1998 Atlantic Geoscience Society Department of Earth Sciences Dalhousie University 1236 Henry Street, Halifax Nova Scotia, Canada B3H3J5 This book was produced with help from The Canadian Geological Foundation, The Department of Earth Sciences, Dalhousie University, and The Atlantic Geoscience Society. ISBN 0-9696009-9-2 AGS Special Publication Number . 14.. I invite you to join the Atlantic Geoscience Society, write clo The Department of Earth Sciences, Dalhousie University (see above) Cover Photo Cape Split looking west into the Minas Channel, Nova Scotia. The split is caused by erosion along North-South faults cutting the Triassic-Jurassic-aged North Mountain Basalt and is the terminal point of a favoured hike of geologists and non-geologists alike. Photo courtesy of Rob • Fensome, Biostratigrapher, -

APPENDIX I Aboriginal Fishing Licences Information
SCOTIAN BASIN EXPLORATION DRILLING PROJECT – ENVIRONMENTAL IMPACT STATEMENT Appendix I – Aboriginal Fishing Licences Information APPENDIX I Aboriginal Fishing Licences Information Scotia-Fundy Communal Commercial Licences by Aboriginal Organization (2015-2016) Aboriginal Organization Licence Id Species Description Licence Type Description Licence Area Description Licence Subtype Description ACADIA FIRST NATION 300209 ALEWIVES/GASPEREAU CC NON-VESSEL BASED LIMITED TUSKET RIVER ACADIA FIRST NATION 120769 CLAMS, UNSPECIFIED CC NON-VESSEL BASED LIMITED CLAM HARVEST AREA - 3 ACADIA FIRST NATION 120846 CLAMS, UNSPECIFIED CC NON-VESSEL BASED LIMITED CLAM HARVEST AREA - 3 ACADIA FIRST NATION 120981 CLAMS, UNSPECIFIED CC NON-VESSEL BASED LIMITED CLAM HARVEST AREA - 3 ACADIA FIRST NATION 121964 CLAMS, UNSPECIFIED CC NON-VESSEL BASED LIMITED CLAM HARVEST AREA - 3 ACADIA FIRST NATION 122000 CLAMS, UNSPECIFIED CC NON-VESSEL BASED LIMITED CLAM HARVEST AREA - 3 ACADIA FIRST NATION 122046 CLAMS, UNSPECIFIED CC NON-VESSEL BASED LIMITED CLAM HARVEST AREA - 3 ACADIA FIRST NATION 122096 CLAMS, UNSPECIFIED CC NON-VESSEL BASED LIMITED CLAM HARVEST AREA - 4 ACADIA FIRST NATION 122141 CLAMS, UNSPECIFIED CC NON-VESSEL BASED LIMITED CLAM HARVEST AREA - 2 ACADIA FIRST NATION 122184 CLAMS, UNSPECIFIED CC NON-VESSEL BASED LIMITED CLAM HARVEST AREA - 3 ACADIA FIRST NATION 122251 CLAMS, UNSPECIFIED CC NON-VESSEL BASED LIMITED CLAM HARVEST AREA - 4 ACADIA FIRST NATION 338316 CRAB, GREEN CC NON-VESSEL BASED LIMITED ACADIA FIRST NATION 338316 CRAB, GREEN CC NON-VESSEL -

Eastern Baccharis Baccharis Halimifolia
COSEWIC Status Report on Eastern Baccharis Baccharis halimifolia in Canada prepared for COMMITTEE ON THE STATUS OF ENDANGERED WILDLIFE IN CANADA by David Mazerolle and Sean Blaney Atlantic Canada Conservation Data Centre P.O. Box 6416, Sackville, New Brunswick E4L 1C6 DRAFT Funding provided by Environment Canada Submitted [Date] EXECUTIVE SUMMARY Wildlife Species Description and Significance Eastern Baccharis is a densely branched and often multi-stemmed, woody, perennial, salt marsh shrub of the Aster family, typically 1 to 3 meters tall in Canada. The species is deciduous in Canada with alternate gray-green leaves. It is dioecious (male and female flowers on separate individuals), flowering in late summer. Inflorescences of tiny flowers arranged in terminal, leafy-bracted panicles which can be very numerous on larger shrubs. Fruit are light brown achenes with a series of elongated pappus bristles to enhance wind dispersal. These bristles are brilliant white and make the female plants very showy and easy to detect when in fruit in late summer and early fall. In Canada, Eastern Baccharis is rare, localized and 400+ km disjunct from the next nearest occurrence in northern Massachusetts, which could be associated with significant genetic divergence. Eastern Baccharis is the only native representative of its genus and subtribe in Canada and one of very few woody Aster family members native to Canada and is used horticulturally in the south and east United States. Baccharis species contain an array of chemicals used medicinally, including some with potential for cancer treatment, but formal investigation of their properties has been limited. American First Nations have used some species in the treatment of sores and wounds, as antibacterials and emetics. -

Ecoregions and Ecodistricts of Nova Scotia
Ecoregions and Ecodistricts of Nova Scotia K.T. Webb Crops and Livestock Research Centre Research Branch Agriculture and Agri-Food Canada Truro, Nova Scotia I.B. Marshall Indicators and Assessment Office Environmental Quality Branch Environment Canada Hull, Quebec Agriculture and Agri-Food Canada Environment Canada 1999 ~ Minister of Public Works and Government Services Cat. No. A42-65/1999E ISBN 0-662-28206-X Copies of this publication are available from: Crops and Livestock Research Centre Research Branch Agriculture and Agri-Food Canada P.O. Box 550, Banting Annex Nova Scotia Agricultural College Truro, Nova Scotia B2N 5E3 or Indicators and Assessment Office Environmental Quality Branch Environment Canada 351 St. Joseph Blvd. Hull, Quebec KIA OC3 Citation Webb, K.T. and Marshall, LB. 1999. Ecoregions and ecodistricts of Nova Scotia. Crops and Livestock Research Centre, Research Branch, Agriculture and Agri-Food Canada, Truro, Nova Scotia; Indicators and Assessment Office, Environmental Quality Branch, Environment Canada, Hull, Quebec. 39 pp. and 1 map. CONTENTS PREFACE. v PREFACE. ., vi ACKNOWLEDGMENTS . vii IN'TRODUCTION 1 ECOSYSTEMS AND ECOLOGICAL LAND CLASSIFICATION 1 THE NATIONAL ECOLOGICAL FRAMEWORK AND NOVA SCOTIA 3 ECOLOGICAL UNITS . 4 Ecozones . 4 Ecoregions . _ . _ 4 Ecodistricts _ . _ . 4 ECOLOGICAL UNIT DESCRIPTIONS . 6 ATLANTIC MARITIME ECOZONE . _ . 6 MARITIME LOWLANDS ECOREGION (122) . 7 Pictou-Cumberland Lowlands Ecodistrict (504) . _ 7 FUNDY COAST ECOREGION (123) _ . 8 Chignecto-Minas Shore Ecodistrict (507) . _ . 9 North MountainEcodistrict(509) _ . _ 10 SOUTHWEST NOVA SCOTIA UPLANDS ECOREGION (124) 11 South Mountain Ecodistrict (510) 12 Chester Ecodistrict (511) . 12 Lunenburg Drumlins Ecodistrict (512) . 13 Tusket River Ecodistrict (513) . -

Yellow Lampmussel (Lampsilis Cariosa) in New Brunswick: a Population of Significant Conservation Value
COSEWIC Assessment and Status Report on the Yellow Lampmussel Lampsilis cariosa in Canada SPECIAL CONCERN 2004 COSEWIC COSEPAC COMMITTEE ON THE STATUS OF COMITÉ SUR LA SITUATION ENDANGERED WILDLIFE DES ESPÈCES EN PÉRIL IN CANADA AU CANADA COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC 2004. COSEWIC assessment and status report on the yellow lampmussel Lampsilis cariosa in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vii + 35 pp. (www.sararegistry.gc.ca/status/status_e.cfm). Production note: COSEWIC acknowledges Derek S. Davis, Kellie L. White and Donald F. McAlpine for writing the status report on the yellow lampmussel Lampsilis cariosa in Canada. The report was overseen and edited by Gerry Mackie, COSEWIC Molluscs Species Specialist Subcommittee Co-chair. COSEWIC also gratefully acknowledges the financial support of the New Brunswick Department of Natural Resources and the Nova Scotia Department of Natural Resources for partially funding the preparation of this status report and providing funding for field travel. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: (819) 997-4991 / (819) 953-3215 Fax: (819) 994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Ếgalement disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur le lampsile jaune (Lampsilis cariosa) au Canada. Cover illustration: Yellow lampmussel — Provided by the author. Her Majesty the Queen in Right of Canada 2004 Catalogue No. -

Recognizing Achievements, Celebrating Success Appendix A
Tides of Change Across the Gulf : Chapter 7 - Recognizing Achievements, Celebrating Success Appendix A - Background Information on Groups Table of Contents Massachusetts Page Essex County Greenbelt Association Inc. .. 1 Massachusetts Bays Monitoring Program. .. 3 Massachusetts Bay Monitoring Program . 6 Massachusetts Water Resources Authority (MWRA) . .7 New England Aquarium Diver Club . 8 Tufts Centre for Conservation Medicine . .9 Woods Hole Oceanographic Institution . .11 Woods Hole Oceanographic Institution . 15 Maine Bagaduce Watershed Association . .21 Blue Hill Heritage Trust . 22 Casco Bay Estuary Project . 23 Cove Brook Watershed Council/8 Rivers Roundtable . 26 Damariscotta River Association . 27 Downeast Salmon Federation . .29 East Penobscot Bay Environmental Alliance . 31 FairPlay for Harpswell . 32 Friends of Acadia. 34 Friends of Taunton Bay . 35 Georges River Tidewater Association . 37 Island Institute . 39 Marine Environmental Research Institute (MERI)] . 41 Maine Sea Grant/Cooperative Extension marine Extension Team . 48 Quoddy Regional Land Trust . 51 Sheepscot Valley Conservation Association . 53 The Chewonki Foundation . 54 The Lobster Conservancy . .. 56 The Maine Chapter of The Nature Conservancy . 59 The Ocean Conservancy-New England Regional Office . 60 Union River Watershed Coalition. .. .62 University of Southern Maine . 63 Vinalhaven Land Trust . 64 Wells NERR Coastal Training Program . 66 New Hampshire Centre for Coastal and Ocean Mapping B University of New Hampshire . 67 Coastal Conservation Association of NH . 68 Nova Scotia Acadia Centre for Estuarine Research . .69 Bay of Fundy Ecosystem Partnership . 71 Bay of Fundy Ecosystem Partnership/Minas Basin Working Group. 73 Bedford Institute of Oceanography . 75 Centre for Water Resources Studies, Dalhousie University . 76 Clean Annapolis River Project . 77 Dr. Arthur Hines School . -
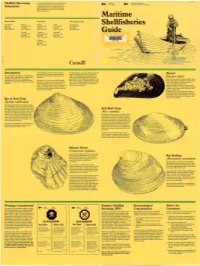
Shellfish Harvesting Information
Shellfish Harvesting It is very important before you col lect any shell Fishe ries Environmen t Canada fish that you ensure the area is not closed . and Oceans Environmental Protection Service Information A check is as simple as using the local tele phone directory to find the federal fisheries •• •• office nearest you or ca ll one of the central offices listed below. Maritime New Brunswick Nova Scotia Prince Edward Island St. Andrews Moncion Sydney Halifax Charlottetown Shellfisheries Box 210 P.O . Box 5030 P.O. Box 1085 P 0 . Box 550 P.O. Box 1236 EOG 2XO E1C 9B6 B1 p 6J7 B3J 2S7 C1A 7M8 (506) 529-8847 (506) 758-9044 (902) 564-7276 (902) 426-24 73 (902) 566-7800 Tracadie Yarmouth Antigonish Guide .,. P.O. Box 1670 215 Main St reet P.O. Box 1183 EOC 2BO B5A 1C6 B2G 2M5 (506) 395-6321 (902) 742-9122 (902) 863-5670 Liverpool P.O. Box 190 BOT 1KO (902) 354-3459 Canada Introduction Fresh shellfish can be purchased at any of As with an y food , care must be taken to ensure hundreds of fish markets , and are served at th at the shellfish gathered are not contam Mussel One of the great attractions of Canada's Mari restaurants and snack bars throughout the inated. This guide has been prepared to pro time provinces is the selection of seafood del Maritimes. vide the recreational digger with information (Mytilus edulis) icacies to be enjoyed here. Among the pertaining to the safe harvest of shellfish in the Rocky shores along the three provinces' coast favourites are the many varieties of shellfish In search of a recreational outing ? Shellfish Maritimes.