Note to Users
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cv1p0149.Pdf
A Publication of Reliable Methods for the Preparation of Organic Compounds Working with Hazardous Chemicals The procedures in Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at http://www.nap.edu/catalog.php?record_id=12654). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices. In some articles in Organic Syntheses, chemical-specific hazards are highlighted in red “Caution Notes” within a procedure. It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure. Prior to performing a reaction, a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for carrying out a risk assessment and for analyzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices. The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein. -

Multicatalytic, Asymmetric Michael/Stetter Reaction Of
Multicatalytic, asymmetric Michael/Stetter reaction SPECIAL FEATURE of salicylaldehydes and activated alkynes Claire M. Filloux, Stephen P. Lathrop, and Tomislav Rovis1 Department of Chemistry, Colorado State University, Fort Collins, CO 80523 Edited by David W. C. MacMillan, Princeton University, Princeton, NJ, and accepted by the Editorial Board May 30, 2010 (received for review March 22, 2010) We report the development of a multicatalytic, one-pot, asymmetric Ar Ar Michael/Stetter reaction between salicylaldehydes and electron- N H deficient alkynes. The cascade proceeds via amine-mediated OO 3 OTMS (20 mol %) Me HO Me Michael addition followed by an N-heterocyclic carbene-promoted Me Me 1 Ar = 3,5-(CF3)2C6H3 O intramolecular Stetter reaction. A variety of salicylaldehydes, dou- + O O BF4 N bly activated alkynes, and terminal, electrophilic allenes participate 2 Me 5 Me in a one-step or two-step protocol to give a variety of benzofura- NNC F 4a 6 5 One-Pot Two - Po t none products in moderate to good yields and good to excellent (10 mol %) 93% yield 46% yield NaOAc (10 mol %) 85:15:<1:<1 dr 5:1 dr enantioselectivities. The origin of enantioselectivity in the reaction 86% ee 58% ee CHCl3, 23 C is also explored; E∕Z geometry of the reaction intermediate as well as the presence of catalytic amounts of catechol additive are O found to influence reaction enantioselectivity. O Me * O Me ∣ ∣ catalysis organic synthesis tandem catalysis Me 6 ascade catalysis has garnered significant recent attention Scheme 1. Multicatalytic Michael/Benzoin cascade. Cfrom the synthetic community as a means to swiftly assemble CHEMISTRY complex molecules from simple starting materials with minimal benzofuranones. -

Synthesis and Antidepressant Activity of Some New Coumarin Derivatives
Scientia Pharmaceutica (Sci. Pharm.) 74, 193-216 (2005) O Osterreichische Apotheker-Verlagsgesellschafl m. b. H., Wien, Printed in Austria Synthesis and Antidepressant Activity of Some New Coumarin Derivatives Nehad A. Abdel-Latif Natural Compounds Department, National Research Centre, Dokki, Cairo, Egypt; E-mail: [email protected] Abstract The coumarin-3-cinnamoyl derivatives 2a-d were prepared via Claisen- Schmidt condensation of 3-acetylcoumarin 1 with different aromatic aldehydes. Cycloaddition reaction of 2b,e with guanidine and thiourea yielded the corresponding aminopyrmimidine 3a,b and thioxypyrimidine derivatives 4a,b, respectively. Compounds 4a,b were condensed with chloroacetic acid or 3- bromopropionic acid to yield coumarin 3-thiazolo-pyrimidine 5a,b and thiazinopyrimidine 6a,b derivatives, respectively. Compounds 4a,b were condensed with chloroacetic acid and aromatic aldehyde to yield the aryl methylene derivatives 7a,b which could be prepared directly by condensation of compounds 5a,b with aromatic aldehydes. Compounds 2a-e were condensed with malononitrile or ethyl cyano acetate in presence of ammonium acetate to yield cyanopyridine 8a,b and cyanopyridone 9a- d derivatives, respectively, which were prepared by condensation of 3- acetylcoumarin 1, malononitrile or ethylcyanoacetate and aromatic aldehydes in presence of ammonium acetate. Condensation of compounds 2a,b,e with o-phenylenediamine in refluxing ethanol led to the formation of 10a-c as intermediate, followed by cleavage by thermolysis to benzimidazole derivative 11 along with compounds 12a-c as mixture, which were obtained directly by fusion of a,&unsaturated ketones 2 with o- phenylene diamine at 200-220°C, while compound 11 could be prepared in pure form by fusion of 1 with o-phenylene diamine at the same temperature. -

Part I: Synthesis of Functionalized Bile Acids Towards The
PART I: SYNTHESIS OF FUNCTIONALIZED BILE ACIDS TOWARDS THE CONSTRUCTION OF STEROIDAL MACROCYCLES. PART II: EVALUATION AND EXPANSION OF A MODULAR CARD GAME FOR TEACHING ORGANIC CHEMISTRY A DISSERTATION IN Chemistry and Pharmaceutical Sciences Presented to the Faculty of the University of Missouri-Kansas City in partial fulfillment of the requirements for the degree DOCTOR OF PHILOSOPHY by CHRISTOPHER ANTON KNUDTSON B.S., Saint Louis University 2004 M.S., Kansas University 2011 Kansas City, Missouri 2019 © 2019 CHRISTOPHER ANTON KNUDTSON ALL RIGHTS RESERVED PART I: SYNTHESIS OF FUNCTIONALIZED BILE ACIDS TOWARDS THE CONSTRUCTION OF STEROIDAL MACROCYCLES. PART II: EVALUATION AND EXPANSION OF A MODULAR CARD GAME FOR TEACHING ORGANIC CHEMISTRY Christopher A. Knudtson, Candidate for the Doctor of Philosophy Degree University of Missouri-Kansas City, 2019 ABSTRACT In Part I, the synthesis of chenodeoxychoic acid (CDCA) derivatives towards the construction of unique chemical architectures are reported. Building on previously described methods, CDCA based macrocycles with inner cavities have been synthesized and characterized. Attempts toward oxa-Michael coupling of CDCA monomers to form sulfonate ester linked dimmers is described. CDCA was also functionalized with terminal alkynes (pentynyl, hexynyl, and heptynyl) and aryl iodide and cyclized to from the respective macrocycles. These have been coupled together via oxygen-free Sonogashira reaction conditions to form large barrel-like steroidal architectures. These structures were investigated by 1H NMR, 13 C NMR spectroscopy, and HR-MS, and MALDI-TOF spectroscopy. In part II a game to teach organic chemistry, ChemKarta was evaluated as a teaching tool. Analysis of an undergraduate class’s impressions of the game showed that Chemkarta was an easy to learn and enjoyable game, but the amount of information in the game could be overwhelming for some students. -

Trusscore Chemical Resistance Chart
Chemical Resistance Chart Table courtesy of JENSEN INERT PRODUCTS, INC. Resistance Material E = Excellent G = Good F = Fair PVC = Polyvinylchloride N = Not Recommended This information, based on experience to date, is believed to be reliable. It is intended as a guide for use at your own discretion and risk. All indications refer to room temperature. Chemical PVC Chemical PVC Acetaldehyde G Calcium Hypochlorite G Acetamide N Carbazole N Acetic Acid, 5% E Carbon Disulfide N Acetic Acid, 50% E Carbon Tetrachloride G Acetone E Chlorine E Aluminum Hydroxide E Chloroacetic Acid F Ammonia E Chloroform N Ammonium Hydroxide E Chromic Acid E Ammonium Oxalate E Citric Acid G n-Amyl Acetate F Cresol N Amyl Chloride N Cyclohexane G Aniline N Chemical PVC Chemical PVC Decalin E Benzaldehyde N o-Dichlorobenzene G Benzene N p-Dichlorobenzene N Benzoic Acid, Sat. E Diethyl Benzene N Benzyl Acetate F Diethyl Ether F Boric Acid E Diethyl Ketone N Bromine G Diethyl Malonate G Bromobenzene F Dimethyl Formamide F n-Butyl Acetate N sec-Butyl Alcohol G Chemical PVC Butyric Acid G Ether F Ethyl Acetate F Chemical PVC Ethyl Benzene N Nitric Acid, 50% G Ethyl Benzoate N Nitric Acid, 70% F Ethyl Butyrate N Nitrobenzene N Ethyl Chloride, Liquid N n-Octane F Ethyl Cyanoacetate NF Orange Oil F Ethyl Lactate F Ethylene Chloride N Chemical PVC Ethylene Glycol E Perchloric Acid G Ethylene Oxide F Perchloroethylene N Phenol, Crystals F Chemical PVC Phosphoric Acid, 1-5% E Fluorine N Phosphoric Acid, 85% E Formic Acid, 50% G Potassium Hydroxide E Formic Acid, 90-100% F Propane Gas E Fuel Oil E Propylene Glycol F Gasoline G Propylene Oxide F Glycerine E Chemical PVC Chemical PVC Resorcinol F n-Heptane F Salicylaldehyde F Hexane G Sulfuric Acid, 1-6% E Hydrochloric Acid, 1-5% E Sulfuric Acid, 20% E Hydrochloric Acid, 35% G Sulfuric Acid, 60% E Hydrofluoric Acid, 4% G Sulfuric Acid, 98% N Hydrofluoric Acid, 48% G Sulfur Dioxide, Liq. -

United States Patent Office Patented Jan
United States Patent Office Patented Jan. 23, 1968 1. 2 3,365,500 Therefore, from what information appears in the litera HYDROXYBENZALDEHYDE PROCESS ture, there would seem to be little advantage to be gained Donald F. Pontz, Midland, Mich., assignor to The Dow by using aqueous ethyl alcohol as a solvent in the prepara Chemical Company, Midland, Mich., a corporation of tion of Salicylaldehyde by this method. There is nothing Delaware to incline a chemist to investigate other alcoholic solvents No Drawing. Filed Jan. 21, 1964, Ser. No. 339,104 as the means by which improved results might be obtained. 5 Claims. (C. 260-600) Consequently, it is surprising and unexpected to find that aqueous methanol used as the solvent medium for the preparation of Salicylaldehyde by the Reimer-Tiemann ABSTRACT OF THE DISCLosURE 10 reaction provides both better combined yields of hy In the Reimer-Tiemann reaction wherein phenol is droxyaldehydes and sharply reduced tar formation as com reacted with chloroform and an alkali metal base to make pared to the results obtained by using either water or salicylaldehyde and p-hydroxybenzaldehyde, improved aqueous ethyl alcohol as the reaction solvent. By using yields of total aldehydes and decreased tar formation are as the Solvent aqueous methanol containing from about obtained when the reaction medium is aqueous methanol 5 10% to about 75% by weight of methanol, the yield of of 10-75 percent by weight concentration as compared Salicylaldehyde is at least as good as that obtained with to reactions run in water alone or in aqueous ethanol. The Water or aqueous ethyl alcohol and the yield of the improvement in total aldehyde yield is due principally to valuable p-hydroxybenzaldehyde is considerably better. -

Fluorescent Iridium(III) Coumarin-Salicylaldehyde Schiff Base Compounds As Lysosome-Targeted Antitumor Agents
Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is © The Royal Society of Chemistry 2020 Fluorescent Iridium(III) Coumarin-salicylaldehyde Schiff Base Compounds as Lysosome-Targeted antitumor agents Cong Liu, Xicheng Liu*, Xingxing Ge, Qinghui Wang, Lei Zhang, Wenjing Shang, Yue Zhang, XiangAi Yuan, Laijin Tian, Zhe Liu*, Jinmao You Institute of Anticancer Agents Development and Theranostic Application, The Key Laboratory of Life-Organic Analysis and Key Laboratory of Pharmaceutical Intermediates and Analysis of Natural Medicine, School of Chemistry and Chemical Engineering, Qufu Normal University, Qufu 273165, China. *Corresponding authors (Email): [email protected] (X. C. Liu); [email protected] (Z. Liu) Supporting Information Experimental Section .....................................................................................................................2 Synthesis.........................................................................................................................................6 Figures S1-S16..........................................................................................................................7 Tables S1-S9...................................................................................................................................24 1 EXPERIMENTAL SECTION NMR Spectrum NMR spectra were acquired in 5 mm NMR tubes at 298 K on Bruker DPX 500 (1H NMR: 500.13 MHz; 13C NMR: 126 MHz; 19F NMR: 471 MHz) spectrometers. 1H NMR chemical shifts were internally referenced -

Role of Nicotinamide and Salicylaldehyde on Some Growth Parameters in Wheat
©Verlag Ferdinand Berger & Söhne Ges.m.b.H., Horn, Austria, download unter www.biologiezentrum.at Phyton (Austria) Vol. 29 Fasc. 1 33-40 16. 5. 1989 Role of Nicotinamide and Salicylaldehyde on Some Growth Parameters in Wheat Youssef A. H. MOHAMED, Ahmed SHARAF EL-DIN & El-Sayed FODA With 3 Figures Received January 13, 1988 Key words: Nicotinamide, salicylaldehyde, growth, wheat, Triticum. Summary MOHAMED Y. A. H., SHARAF EL-DIN A. & FODA E. 1989. Role of nicotinamide and salicylaldehyde on some growth parameters in wheat. - Phyton (Austria) 29 (1): 33-40, with 3 figures. - English with German summary. The effect of 1(T4 and 1CT5 M nicotinamide and salicylaldehyde was shown in favour of the increase in root and shoot length. The area of the leaf in plants treated with nicotinamide increased and the width decreased. Salicylaldehyde exerted neu- tral effect on leaf. Both growth regulators increased the level of total soluble carbohydrates and total soluble proteins throughout the overall age of the plant and in the different plant organs. As a result of these growth regulators application, DNA quantities decreased in the root and stem and increased in the leaf, while inhibition of RNA synthesis was observed. Zusammenfassung MOHAMED Y. A. H., SHARAF EL-DIN A. & FODA E. 1989. Der Einfluß von Nikotinamid und Salicylaldehyd auf einige Wachstumsparameter bei Weizen. - Phyton (Austria) 29 (1): 33-40, mit 3 Figuren. - Englisch mit deutscher Zusammen- fassung. 10"4 und 10~5 M Nikotinamid und Salicylaldehyd begünstigten das Längen- wachstum von Wurzel und Sproß. Die Blattfläche nikotinamidbehandelter Pflanzen nahm zu, während deren Breite abnahm. -

Synthetic and Meschanistic Studies of The
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by South East Academic Libraries System (SEALS) APPLICATIONS OF THE BAYLIS-HILLMAN REACTION IN THE SYNTHESIS OF COUMARIN DERIVATIVES A thesis submitted in fulfilment of the requirements for the degree DOCTOR OF PHILOSOPHY of RHODES UNIVERSITY by MUSILIYU AYODELE MUSA M.Sc (Lagos) Department of Chemistry Rhodes University Grahamstown January 2002 ABSTRACT The reaction of specially prepared salicylaldehyde benzyl ethers with the activated alkenes, methyl acrylate or acrylonitrile, in the presence of the catalyst, DABCO, has afforded Baylis-Hillman products, which have been subjected to conjugate addition with either piperidine or benzylamine. Hydrogenolysis of these conjugate addition products in the presence of a palladium-on-carbon catalyst has been shown to afford the corresponding 3-substituted coumarins, while treatment of O-benzylated Baylis- Hillman adducts with HCl or HI afforded the corresponding 3-(halomethyl)coumarins directly, in up to 94%. The 3-(halomethyl)coumarins have also been obtained in excellent yields (up to 98%) and even more conveniently, by treating the unprotected Baylis-Hillman products with HCl in a mixture of AcOH and Ac2O, obtained from tert-butyl acrylate and various salicylaldehydes. The generality of an established route to the synthesis of coumarins via an intramolecular Baylis-Hillman reaction, involving the use of salicylaldehyde acrylate esters in the presence of DABCO, has also been demonstrated. Reactions between the 3-(halomethyl)coumarins and various nitrogen and carbon nucleophiles have been shown to proceed with a high degree of regioselectivity at the exocyclic allylic centre to afford 3-substituted coumarin products. -

Summary: Synthesis of Salicylaldehyde and Its Applications Twghs Kap Yan Directors’ College Introduction
Summary: Synthesis of Salicylaldehyde and its Applications TWGHs Kap Yan Directors’ College Introduction Salicylaldehyde (SA) (C6H4CHO-2-OH) is a useful aromatic organic chemical. It is a common reactant for many synthesis, such as cyclic organic compounds like coumarin and organic ligand such as salan ligand. Salicylaldehyde is also crucial to our daily lives. In A. Ravin’s medical publication, it is mentioned that SA can be used for detection of ketonuria in medical treatment. It also serves as a chelating agent with heavy metal ions such as Copper(II) ion, which is applicable to colorimetric analysis of the concentration of heavy metal ions in water sample. SA is also a precursor in total synthesis of coumarin (C9H6O2), which is a white organic crystal with antibacterial activities. However, salicylaldehyde is not commonly stored in secondary schools’ laboratories. People can only buy a relatively large amount of salicylaldehyde with high price from chemical suppliers. In our research project, we used a simple one-pot reaction to synthesize salicylaldehyde from phenol (C6H5OH), sodium hydroxide (NaOH) and chloroform (CHCl3) which are available in school laboratory. The synthesis, separation and identification are carried under a normal secondary school laboratory without using advanced laboratory devices and apparatus. Meanwhile, several applications of salicylaldehyde were also investigated, for instance the synthesis of coumarin, metal complex chelation and the medical detection of ketonuria. Figure 1: Chemical structure of salicylaldehyde Figure 2: Appearance of salicylaldehyde Part 1: Synthesis By Reimer-Tiemann Reaction, phenol reacts with chloroform to form SA under refluxing for 1 hour in 65℃ water bath. This method is simple to carry out with a relatively high yield of SA obtained (~30%). -

Bis(Salicylidene)Ethylenediamine(Salen) and Bis(Salicylidene)Ethylenediamine-Metal Complexes: from Structure to Biological Activity
Journal of Analytical & Pharmaceutical Research Bis(Salicylidene)Ethylenediamine(Salen) and Bis(Salicylidene)Ethylenediamine-Metal Complexes: from Structure to Biological Activity Abstract Review Article Schiff bases and their metal complexes have occupied a central role in the Volume 3 Issue 6 - 2016 development of co-ordination chemistry as evidenced by the vast number, ease and flexibility of the synthetic procedure, diverse properties, and use as biologically active compounds as antitumor, antibacterial, antifungal and other miscellaneous Department of Industrial Chemistry, Ebonyi State University, biological applications. Many reports have demonstrated that Salen and Salen- Nigeria metal complexes are highly active against several diseases, including cancer. The thrust of this review work is to evaluate the structural nature of Salen and assess *Corresponding author: Felix Sunday Nworie, Department the biological applications thereof. of Industrial Chemistry, Ebonyi State University, PMB 053 Abakaliki, Ebonyi State, Nigeria, Tel: +2348034813342; Keywords: Schiff base; Salen; Metal salen; Biological applications; Structural Email: characterization Received: October 31, 2016 | Published: December 15, 2016 Abbreviations: C: Carbon; N: Nitrogen; DMSO: Dimethylsulphoxide; PXRD: Powder X-ray Diffractometer; the elimination of the hemiaminal (carbinolamine) hydroxyl SOD: Superoxide Dismutase; Cu: Copper; Zn: Zinc; Fe: Iron; groupconditions, (dehydration there is unavailabilitystep) [5]. of sufficient protons to catalyze Cr: Chromium; Mn: -
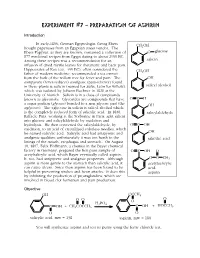
Experiment 7
Experiment #7 – Preparation of Aspirin Introduction In early-1800, German Egyptologist Georg Ebers CH2OH bought papyruses from an Egyptian street vendor. The Ebers Papyrus, as they are known, contained a collection of O glucose 877 medicinal recipes from Egypt dating to about 2500 BC. Among these recipes was a recommendation for an salicin infusion of dried myrtle leaves for rheumatic and back pain. Hippocrates of Kos (ca. 400 BC), often considered the CH2OH father of modern medicine, recommended a tea extract from the bark of the willow tree for fever and pain. The OH antipyretic (fever-reducer) analgesic (pain-reliever) found in these plants is salicin (named for Salix, Latin for willow), salicyl alcohol which was isolated by Johann Buchner in 1828 at the University of Munich. Salicin is in a class of compounds known as glycosides. Glycosides are compounds that have OHC a sugar portion (glycose) bonded to a non-glycose part (the OH aglycone). The aglycone in salicin is salicyl alcohol which is the completely reduced form of salicylic acid. In 1838, salicylaldehyde Raffaele Piria, working at the Sorbonne in Paris, split salicin into glucose and salicylaldehyde by oxidation and hydrolysis. He then converted the salicylaldehyde, by OOHC oxidation, to an acid of crystallized colorless needles, which he named salicylic acid. Salicylic acid had antipyretic and OH analgesic qualities; unfortunately it was too harsh to the salicylic acid linings of the mouth, esophagus and stomach. On August 10, 1897, Felix Hoffmann, a chemist in the Bayer chemical factory in Germany, prepared the first pure sample of OOHC O acetylsalicylic acid, which Bayer eventually called aspirin.