Habitat Restoration and GIS Mapping of Rana Aurora and Monadenia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

San Gabriel Chestnut ESA Petition
BEFORE THE SECRETARY OF THE INTERIOR PETITION TO THE U.S. FISH AND WILDLIFE SERVICE TO PROTECT THE SAN GABRIEL CHESTNUT SNAIL UNDER THE ENDANGERED SPECIES ACT © James Bailey CENTER FOR BIOLOGICAL DIVERSITY Notice of Petition Ryan Zinke, Secretary U.S. Department of the Interior 1849 C Street NW Washington, D.C. 20240 [email protected] Greg Sheehan, Acting Director U.S. Fish and Wildlife Service 1849 C Street NW Washington, D.C. 20240 [email protected] Paul Souza, Director Region 8 U.S. Fish and Wildlife Service Pacific Southwest Region 2800 Cottage Way Sacramento, CA 95825 [email protected] Petitioner The Center for Biological Diversity is a national, nonprofit conservation organization with more than 1.3 million members and supporters dedicated to the protection of endangered species and wild places. http://www.biologicaldiversity.org Failure to grant the requested petition will adversely affect the aesthetic, recreational, commercial, research, and scientific interests of the petitioning organization’s members and the people of the United States. Morally, aesthetically, recreationally, and commercially, the public shows increasing concern for wild ecosystems and for biodiversity in general. 1 November 13, 2017 Dear Mr. Zinke: Pursuant to Section 4(b) of the Endangered Species Act (“ESA”), 16 U.S.C. §1533(b), Section 553(3) of the Administrative Procedures Act, 5 U.S.C. § 553(e), and 50 C.F.R. §424.14(a), the Center for Biological Diversity and Tierra Curry hereby formally petition the Secretary of the Interior, through the United States Fish and Wildlife Service (“FWS”, “the Service”) to list the San Gabriel chestnut snail (Glyptostoma gabrielense) as a threatened or endangered species under the Endangered Species Act and to designate critical habitat concurrently with listing. -

Conservation Assessment for Cryptomastix Hendersoni
Conservation Assessment for Cryptomastix hendersoni, Columbia Oregonian Cryptomastix hendersoni, photograph by Bill Leonard, used with permission. Originally issued as Management Recommendations February 1999 by John S. Applegarth Revised Sept 2005 by Nancy Duncan Updated April 2015 By: Sarah Foltz Jordan & Scott Hoffman Black (Xerces Society) Reviewed by: Tom Burke USDA Forest Service Region 6 and USDI Bureau of Land Management, Oregon and Washington Interagency Special Status and Sensitive Species Program Cryptomastix hendersoni - Page 1 Preface Summary of 2015 update: The framework of the original document was reformatted to more closely conform to the standards for the Forest Service and BLM for Conservation Assessment development in Oregon and Washington. Additions to this version of the Assessment include NatureServe ranks, photographs of the species, and Oregon/Washington distribution maps based on the record database that was compiled/updated in 2014. Distribution, habitat, life history, taxonomic information, and other sections in the Assessment have been updated to reflect new data and information that has become available since earlier versions of this document were produced. A textual summary of records that have been gathered between 2005 and 2014 is provided, including number and location of new records, any noteworthy range extensions, and any new documentations on FS/BLM land units. A complete assessment of the species’ occurrence on Forest Service and BLM lands in Oregon and Washington is also provided, including relative abundance on each unit. Cryptomastix hendersoni - Page 2 Table of Contents Preface 1 Executive Summary 4 I. Introduction 6 A. Goal 6 B. Scope 6 C. Management Status 6 II. Classification and Description 7 A. -

Barred Owls to Benefit Threatened Northern Spotted Owls
Experimental Removal of Barred Owls to Benefit Threatened Northern Spotted Owls Draft Environmental Impact Statement Prepared by Oregon Fish and Wildlife Office U.S. Fish and Wildlife Service Portland, Oregon March, 2012 Experimental Removal of Barred Owls to Benefit Threatened Northern Spotted Owls Draft EIS Table of Contents Table of Contents ............................................................................................................... i List of Tables .................................................................................................................. xiii List of Figures ............................................................................................................... xviii Executive Summary ....................................................................................................... xxi S.1 Background ............................................................................................................ xxi S.2 Purpose of and Need for the Action .................................................................... xxiii S.3 Description of the Proposed Action ..................................................................... xxiii S.4 Considerations Used in Developing the Alternatives .......................................... xxiv S.4.1 Number of Study Areas ................................................................................ xxiv S.4.2 Distribution of Study Areas .......................................................................... xxiv S.4.3 Type of Study -

Gastropoda E Bivalvia.Key
Mollusca 1 Quem são: Mexilhões, lulas, polvos, caramujos, lesmas etc. Cerca de 100.000 espécies viventes descritas e 70.000 espécies fósseis. 2 Aplacophora 3 Monoplacophora 4 Polyplacophora 5 Gastropoda 6 Bivalvia 7 Cephalopoda 8 Scaphopoda 9 LETTER RESEARCH , , yielded nearly identical topologies within Mollusca, except for relation-1 1–3,10 ships among basal gastropods and placements of the sea slug oxidase I c Gastropoda Pleurobranchaea and the bivalve Mytilus (Fig. 2 and Supplementary Pleistomollusca , Unnamed f , Scott R. Santos Fig. 5). High leaf stability scores for all OTUs (Supplementary Table4 3) . doi:10.1038/nature10382 10 a . Moreover, Mollusca was and strong support for most nodes suggest all OTUs were represented 3 Bivalvia Aculifer Institute of Zoology, Johannes Gutenberg by sufficient data to be reliably placed. Remarkably, branch lengths 4 . Unfortunately, because of varying 6 , Achim Meyer 3 were relatively uniform; cephalopods did not show long branches as Conchifera recovered most major lineages mono- 1–3,10 1 previously reported in analyses of 18S and 28S . Scaphopoda 00 MONTH 2011 | VOL 000 | NATURE | 1 Scaphopoda Bivalvia Monoplacophora Gastropoda Cephalopoda . Morphology has been interpreted to divide Neomeniomorpha Chaetodermomorpha Polyplacophora Conchifera 4 has been widely debated. Some workers have con- 1 4,9 All major lineages of Mollusca were monophyletic with strong , Diasoma and Cyrtosoma hypotheses allying bivalves to yielded similar results, namely that bivalves were not e 2 , Andrea B. Kohn 5 5 f support (bs 100%, pp 1.00). Importantly, there was strong3 Cephalopodaa Department of Biology and Centre for Geobiology, University of Bergen, P.O. Box 7800, NO- support at all deep nodes, although the node placing Scaphopoda Mollusca 2 Testaria Morphological and traditional molecular phylogenetic approaches Molecular investigations of molluscan phylogeny have relied Cyrtosoma Diasoma have failed to robustly reconstruct mollusc phylogeny. -

Rare, Threatened and Endangered Invertebrate Species of Oregon
Rare, Threatened and Endangered Invertebrate Species of Oregon August 2016 Oregon Biodiversity Information Center, Portland, Oregon Scientific Name Ecoregion; Adjacent States Heritage Federal ODFW ORBIC Common Name Oregon Counties Rank Status Status List INVERTEBRATES Class Turbellaria - Flatworms Order Tricladida Kenkia rhynchida BR G1G2 SOC-- 1 A flatworm (planarian) Harn S1S2 CS Class Bivalvia - Clams, Oysters and Mussels Order Ostreoida Ostrea conchaphila CR, ME; WA G5 -- -- 3 Native oyster Linc, Till SNR Order Unionoida Anodonta californiensis BM, BR, CB, CR, EC, WC, WV; CA, ID, NV, WA + G3Q SOC-- 3 California floater (mussel) Clat, Colu, Coos?, Desc, Gran, Harn, Klam, Linn, Malh, S2 CS Mult, Sher, Wasc, Wash Anodonta nuttalliana BM, BR, CB, CR, EC?, KM, WC, WV; BC, CA, ID, NV, G4Q -- -- 3 Winged floater (mussel) WA + S1? CS Bake, Clac, Clat, Colu, Coos, Desc, Doug, Gran, Harn, Jack, Jeff, Jose, Klam, Lake, Lane, Linc, Linn, Malh, Morr, Mult, Umat, Wasc?, Wash, Whee Anodonta oregonensis BM, BR, CB, CR, EC, KM, WC, WV; AK, BC, CA, ID, G5Q -- -- 2 Oregon floater (mussel) NV, UT, WA S3? Bake, Bent, Clac, Clat, Colu, Coos, Doug, Gran, Harn, Hood, Jack, Klam, Lake, Lane, Linc, Linn, Mari, Mult, Polk, Sher, Umat, Wasc, Wash, Yamh Gonidea angulata BM, BR, CB, CR, EC, KM, WC, WV; BC, CA, ID, NV, G3 -- -- 2 Western ridged mussel WA + S2S3 CS Bake, Bent, Clac, Colu, Coos, Croo, Curr, Desc, Doug, Gran, Harn, Jeff, Jose, Klam, Linn, Malh, Mari, Morr, Mult, Umat, Unio, Wall, Wasc, Wash, Whee, Yamh Margaritifera falcata BM, BR, CB, CR, EC, KM, WC, WV; AK, BC, CA, ID, G5 -- -- 2 Western pearlshell (mussel) NV, WA + S3 Bake, Bent, Clac, Clat, Colu, Coos, Croo, Curr, Desc, Doug, Gran, Harn, Hood, Jack, Jeff, Jose, Klam, Lake, Lane, Linc, Linn, Malh, Mari, Morr, Mult, Polk, Sher, Till, Umat, Unio, Wall, Wasc, Wash, Whee, Yamh Order Veneroida Pisidium sp. -

Competitive Interactions and Resource Partitioning Between Northern Spotted Owls and Barred Owls in Western Oregon
Competitive interactions and resource partitioning between northern spotted owls and barred owls in western Oregon Wiens, J. D., Anthony, R. G. and Forsman, E. D. (2014), Competitive interactions and resource partitioning between northern spotted owls and barred owls in western Oregon. Wildlife Monographs, 185: 1–50. doi:10.1002/wmon.1009 10.1002/wmon.1009 John Wiley & Sons, Ltd. Version of Record http://hdl.handle.net/1957/48214 http://cdss.library.oregonstate.edu/sa-termsofuse Wildlife Monographs 185:1–50; 2014; DOI: 10.1002/wmon.1009 Competitive Interactions and Resource Partitioning Between Northern Spotted Owls and Barred Owls in Western Oregon J. DAVID WIENS,1 U.S. Geological Survey Forest and Rangeland Ecosystem Science Center, Department of Fisheries and Wildlife, Oregon Cooperative Fish and Wildlife Research Unit, Oregon State University, 3200 SW Jefferson Way, Corvallis, OR 97331, USA ROBERT G. ANTHONY, Department of Fisheries and Wildlife, Oregon Cooperative Fish and Wildlife Research Unit, Oregon State University, Corvallis, OR 97331, USA ERIC D. FORSMAN, USDA Forest Service, Pacific Northwest Research Station, Forestry Sciences Laboratory, Corvallis, OR 97331, USA ABSTRACT The federally threatened northern spotted owl (Strix occidentalis caurina) is the focus of intensive conservation efforts that have led to much forested land being reserved as habitat for the owl and associated wildlife species throughout the Pacific Northwest of the United States. Recently, however, a relatively new threat to spotted owls has emerged in the form of an invasive competitor: the congeneric barred owl (S. varia). As barred owls have rapidly expanded their populations into the entire range of the northern spotted owl, mounting evidence indicates that they are displacing, hybridizing with, and even killing spotted owls. -
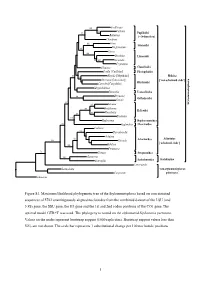
Figure S1. Maximum Likelihood Phylogenetic Tree of The
100 Cochlicopa 55 Vallonia 92 Pupilloidei Buliminus [= Orthurethra] Chondrina Arion 100 Arionoidei 66 Meghimatium Vitrina 100 Oxychilus Limacoidei 82 100 Euconulus Cryptozona Albinaria Clausilioidei Corilla [Corillidae] Plectopyloidea 70 Rhytida [Rhytididae] Helicina 53 Dorcasia [Dorcasiidae] [‘non-achatinoid clade’] Caryodes [Caryodidae] Rhytidoidei Megalobulimus Testacella Testacelloidea Drymaeus 94 Orthalicoidei Gaeotis 82 93 Satsuma Stylommatophora 100 Bradybaena Helicoidei Monadenia 87 93 84 Trochulus Haplotrema Haplotrematoidea 93 Euglandina Oleacinoidea Coeliaxis 92 Thyrophorella Achatina 92 Achatinina 100 Glessula Achatinoidea [‘achatinoid clade’] 100 Subulina Ferussacia 76 Gonaxis Streptaxoidea 100 Guestieria Systrophia Scolodontoidea Scolodontina Laevicaaulis Laemodonta ‘non-stylommatophoran Carychium pulmonates’ Siphonaria 1% 0.01 Figure S1. Maximum likelihood phylogenetic tree of the Stylommatophora based on concatenated sequences of 5782 unambiguously aligned nucleotides from the combined dataset of the LSU (and 5.8S) gene, the SSU gene, the H3 gene and the 1st and 2nd codon positions of the CO1 gene. The optimal model GTR+G was used. The phylogeny is rooted on the siphonariid Siphonaria pectinata. Values on the nodes represent bootstrap support (1000 replicates). Bootstrap support values less than 50% are not shown. The scale bar represents 1 substitutional change per 100 nucleotide positions. 1 91 Satsuma 100 Bradybaena Trochulus 97 Helicoidei 68 Monadenia 87 Haplotrema Haplotrematoidea Euglandina Oleacinoidea 100 Vallonia -

Habitat Assessment of the Pacific Sideband (Monadenia Fidelis) in the Lower Fraser Valley British Columbia
Pacific Sideband 1 Habitat Assessment of the Pacific Sideband (Monadenia fidelis ) In the Lower Fraser Valley British Columbia December 20, 2007 Darren Brown, BIT Ryan Durand, R.P.Bio. Fraser Valley Conservancy Society Abbotsford, BC Pacific Sideband 2 Executive Summary The Pacific sideband ( Monadenia fidelis ) is the largest land snail known to occur in British Columbia. Although blue-listed in British Columbia for apparent scarcity and threats to its habitat, the snail is widespread throughout the western United States. This report attempts to define the habitat requirement of the Pacific sideband in the lower Fraser Valley; specifically the Abbotsford area and to a lesser degree adjacent municipalities. Data was collected in conjunction with a variety of mapping projects from January 2005 to August 2007. Additional data continues to be collected and may be used to refine this report in the future. Data from 159 sample plots were analyzed to determine a variety of ecological and physical site features of preferred snail habitat. Analysis included physical conditions such as slope, elevation, and substrate, as well as ecological descriptions of dominant vegetation and associations of trees, shrubs, and herbs. In general the results of this study indicate that Pacific sideband is most commonly associated with relatively undisturbed deciduous and mixed forested areas. However, its habitat requirements appeared to be highly variable and we continue to find it in unexpected locations. Pacific Sideband 3 Acknowledgements There are several people who helped with this study. Sincere thanks to Ryan Durand for providing me this incredible opportunity to work with him. Also special thanks to Michelle Scott and Natashia Cox of Taara Environmental who assisted with data collection throughout the project. -

California Status Factors
California Status Factors Elcode IMGASC7037 Gname MONADENIA FIDELIS KLAMATHICA Gcomname OAK FLAT OR KLAMATH SIDEBAND Number of Occurrences B = 6 - 20 Comments Known from 11 sites, all historical except the type locality (along Oak Flat Creek, near the Klamath River, Siskiyou County, California--Burke et al., 1999) and five miles upstream from forks of the Salmon River, Siskiyou County, California (Frest and Johannes, 2000; Roth, 1993). Frest and Johannes (2000) list fewer sites. Number of Occurrences with Good Viability B = Very few (1-3) occurrences with good viability Comments No specimens have been found for many years, except for specimens collected at the type locality in 1998 by T.R. Weasma (Burke et al., 1999). Only one site known currently, five miles upstream from forks of Salmon River, Siskiyou County, California (Frest and Johannes, 2000; Roth, 1993). Population Size U = Unknown Comments Local and range-wide population trends are not known (Burke et al., 1999). Range Extent C = 250-1,000 km2 (about 100-400 square miles) D = 1,000-5,000 km2 (about 400-2,000 square miles) Comments Known from Siskiyou and Humboldt Counties, the Salmon River drainage, possibly down to the Hoopa Valley Indian Reservation, California, but not east of Sacramento or into coastal areas. Known from two sites in Siskiyou and Humboldt Counties, California, in the main Klamath River canyon and possibly also in the Salmon River drainage (Frest and Johannes, 2000). Range does not extend east of the Sacramento River Basin or into coastal areas (Kelley et al., 1999). Monadenia fidelis klamathica is only known from 11 known sites in 3 small areas collected in the 1930s and one in the Salmon River drainage collected in the early 1950s. -

Heritage Rank Status Factors
Heritage Rank Status Factors Elcode IMGASC7037 Gname MONADENIA FIDELIS KLAMATHICA Gcomname OAK FLAT OR KLAMATH SIDEBAND Number of Occurrences B = 6 - 20 Comments Known from 11 sites, all historical except the type locality (along Oak Flat Creek, near the Klamath River, Siskiyou County, California--Burke et al., 1999) and five miles upstream from forks of the Salmon River, Siskiyou County, California (Frest and Johannes, 2000; Roth, 1993). Frest and Johannes (2000) list fewer sites. Number of Occurrences with Good Viability B = Very few (1-3) occurrences with good viability Comments No specimens have been found for many years, except for specimens collected at the type locality in 1998 by T.R. Weasma (Burke et al., 1999). Only one site known currently, five miles upstream from forks of the Salmon River in Siskiyou County, California (Frest and Johannes, 2000; Roth, 1993). Population Size U = Unknown Comments Local and range-wide population trends are not known (Burke et al., 1999). Range Extent C = 250-1,000 km2 (about 100-400 square miles) D = 1,000-5,000 km2 (about 400-2,000 square miles) Comments Known from Siskiyou and Humboldt Counties, the Salmon River drainage, possibly up to Josephine County, Oregon and down to the Hoopa Valley Indian Reservation, California, but not east of Sacramento or into coastal areas. Known from two sites in Siskiyou and Humboldt Counties, California, in the main Klamath River canyon and in the Salmon River drainage (Frest and Johannes, 2000). Range may extend as far north as Josephine County, Oregon, but not east of the Sacramento River Basin or into coastal areas (Kelley et al., 1999). -

(Pyrgulopsis Robusta), Harney Lake Springsnail (Pyrgulopsis Hendersoni), and Columbia Springsnail (Pyrgulopsis New Species 6) As Threatened Or Endangered
PETITION BEFORE THE SECRETARY OF THE INTERIOR Petition to List the Jackson Lake springsnail (Pyrgulopsis robusta), Harney Lake springsnail (Pyrgulopsis hendersoni), and Columbia springsnail (Pyrgulopsis new species 6) as Threatened or Endangered Submitted by: Dr. Peter Bowler, Biodiversity Conservation Alliance, Center for Biological Diversity, Center for Native Ecosystems, Western Watersheds Project, The Xerces Society July 2004 EXECUTIVE SUMMARY Invertebrates comprise nearly 99% of the world’s animal diversity and play an invaluable role in maintaining environmental health and integrity. Invertebrates are a part of nearly every food chain, are responsible for recycling plant and animal waste in soils and waters, and are crucial to ensuring the perpetuation of food webs. Mollusks, invertebrates characterized by their shells and which include snails, slugs, clams, mussels, and other creatures, are one of the most diverse group of animals in the world. Worldwide, it is estimated that 50,000-200,000 different mollusk species exist on land and in freshwater. These species exist in an incredible diversity of environments, playing a vital role in sustaining clean and healthy air, soil, water, and vegetation. In the western United States, which includes the states of Arizona, California, Colorado, Idaho, Montana, Nevada, New Mexico, Oregon, Utah, Washington, and Wyoming, land and freshwater mollusks are probably the most diverse group of animals and comprise an irreplaceable proportion of worldwide diversity. Over a thousand species of snails, slugs, clams, and mussels are found on land and in freshwater. Scientists estimate hundreds more species have yet to be discovered. Some species in the west exist in only one or two locations, such as a desert spring, a stand of ancient forest, or a coastal island, and are found nowhere else in the world. -

Species Fact Sheet for Monadenia Fidelis Flava
SPECIES FACT SHEET Common Name: Green sideband (Pacific sideband) Scientific Name: Monadenia fidelis flava (Hemphill, 1892) (Synonym: Monadenia fidelis beryllica [Chace & Chace, 1935]) Phylum: Mollusca Class: Gastropoda Order: Stylommatophora Family: Bradybaenidae Conservation Status Global Status (2005): G4G5T1T2 (last reviewed 24 Mar 2005) Rounded Global Status: T1 – Critically imperiled Reasons: Rare Oregon endemic (ORBIC, March 2005) National Status (US): N1N2 (24 Mar 2005) State Status (Oregon): S1S2 (NatureServe 2014) IUCN Red List: NE – Not evaluated Taxonomic Note This subspecies was previously known as Monadenia fidelis beryllica Chace & Chace, 1935, but the earlier name of M. f. flava Hemphill, 1892, has been resurrected as the valid name for the subspecies of M. fidelis that occurs along the coast and for some distance inland in Curry County, OR (Roth 2011, pers. comm.). Coan and Roth (1987) note that “the only extant type specimen [for M. f. flava]… is from within the range of M. f. beryllica, and is the earliest available name for that subspecies.” According to Frest and Johannes (2000), this species can also be found under the name Aglaja fidelis var. flavus Hemphill, 1892. Another taxonomic entity, Monadenia fidelis baxteriana Talmadge, 1954, has been described from the immediate vicinity of Sisters Rocks, north of Ophir, in Curry County, OR., but it has a dark brown base, and is smaller (<30 mm) (Talmadge 1954). Talmadge, who originally named the subspecies, decided in a later review (Talmadge 1961) that it was in fact synonymous with M. f. beryllica (now flava). Coan & Roth (1987) agree with this synonymy; however, Frest and Johannes (2000) believe this taxon should stand on its own as a valid subspecies given differences in anatomy and shell morphology.