Biometric Analysis of Interspecific Hybrids Between Rosa Canina L
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rosa L.: Rose, Briar
Q&R genera Layout 1/31/08 12:24 PM Page 974 R Rosaceae—Rose family Rosa L. rose, briar Susan E. Meyer Dr. Meyer is a research ecologist at the USDA Forest Service’s Rocky Mountain Research Station Shrub Sciences Laboratory, Provo, Utah Growth habit, occurrence, and uses. The genus and act as seed dispersers (Gill and Pogge 1974). Wild roses Rosa is found primarily in the North Temperate Zone and are also utilized as browse by many wild and domestic includes about 200 species, with perhaps 20 that are native ungulates. Rose hips are an excellent source of vitamin C to the United States (table 1). Another 12 to 15 rose species and may also be consumed by humans (Densmore and have been introduced for horticultural purposes and are nat- Zasada 1977). Rose oil extracted from the fragrant petals is uralized to varying degrees. The nomenclature of the genus an important constituent of perfume. The principal use of is in a state of flux, making it difficult to number the species roses has clearly been in ornamental horticulture, and most with precision. The roses are erect, clambering, or climbing of the species treated here have been in cultivation for many shrubs with alternate, stipulate, pinnately compound leaves years (Gill and Pogge 1974). that have serrate leaflets. The plants are usually armed with Many roses are pioneer species that colonize distur- prickles or thorns. Many species are capable of clonal bances naturally. The thicket-forming species especially growth from underground rootstocks and tend to form thick- have potential for watershed stabilization and reclamation of ets. -

Gardens R by DANIEL J
gARDENS r BY DANIEL J. FOLEY Editor of Horticulture REPRINTED FROM THE HERBARIST A Publication of The Herb Society of America No. 19 $<X>$«><>$*3><><>3><><>&3>3^^ For Use and for Delight :>>&<X><2><»3>'3K3x3>O<3K><>c?<>3><^ BOSTON, MASSACHUSETTS 1953 MARY GARDENS r c By DANIEL J. FOLEY MORE than a quarter of a century ago when I first began to explore the plant realm, I remember a visit I made one warm afternoon in June. It was to an old Salem garden where sweet William and fox- gloves, delphiniums and Canterbury bells, ferns and sweet rocket and a host of other plants flourished in a series of meandering bor- ders. The flower beds were edged with violets which were kept trim and formal by reason of the " bobbing " or shearing their owner gave them on several occasions through the summer months. I recall an espaliered peach tree which covered one side of the old tool shed, but most of all I remember a figure of Our Lady enshrined in a shady corner of the garden. My inquisitiveness got the better of me and I asked about the shrine. The dear old lady who tended the garden told me that she had dedicated her garden to Mary and, some- how, the thought lingered with me. At that time I knew nothing of the tradition of the Mary Gardens of the Middle Ages, but a few years later, while doing some research in college, I discovered a host of ancient plant traditions associated with the life of Our Lady. -

A Systematic Review on the Rosa Canina Effect and Efficacy Profiles
PHYTOTHERAPY RESEARCH Phytother. Res. 22, 725–733 (2008) Published online 3 April 2008 inROSA Wiley CANINA InterScience EFFECT AND EFFICACY PROFILES 725 (www.interscience.wiley.com) DOI: 10.1002/ptr.2400 REVIEW ARTICLE A Systematic Review on the Rosa canina Effect and Efficacy Profiles Cosima Chrubasik1,2, Basil D. Roufogalis3, Ulf Müller-Ladner2 and Sigrun Chrubasik1,3* 1Department of Forensic Medicine, University of Freiburg, Albertstr. 9, 79104 Freiburg, Germany 2Abteilung für Rheumatologie und Klinische Immunologie, Kerckhoff-Klinik Bad Nauheim/Lehrstuhl für Innere Medizin mit Schwerpunkt Rheumatologie der Justus-Liebig-Universität Giessen, Benekestr. 2-8, D 61231 Bad Nauheim, Germany 3Herbal Medicines Research and Education Centre, Faculty of Pharmacy, University of Sydney, NSW 2006, Australia Rose hip, rose hip and seed and rose hip seed, all were negatively monographed by the German Commission E due to insufficient evidence of effects and effectiveness. Therefore a comprehensive review of the literature was conducted to summarize the pharmacological and clinical effects of Rosa canina L. to reevaluate its usefulness in traditional medicine. For various preparations of rose hip and rose hip and seed, antioxidative and antiinflammatory effects have been demonstrated. Lipophilic constituents are involved in those mechanisms of action. The proprietary rose hip and seed powder LitozinR has been employed successfully in a number of exploratory studies in patients suffering from osteoarthritis, rheumatoid arthritis and low back pain. However, the sizes of the clinical effects for the different indications need to be determined to assure clinical significance. There is also a rationale behind the use of LitozinR as part of a hypocaloric diet based on the rose hip probiotic, stool regulating and smooth muscle-relaxing actions, as well as the rose hip seed lipid-lowering, antiobese and antiulcerogenic effects. -

Taxonomic Review of the Genus Rosa
REVIEW ARTICLE Taxonomic Review of the Genus Rosa Nikola TOMLJENOVIĆ 1 ( ) Ivan PEJIĆ 2 Summary Species of the genus Rosa have always been known for their beauty, healing properties and nutritional value. Since only a small number of properties had been studied, attempts to classify and systematize roses until the 16th century did not give any results. Botanists of the 17th and 18th century paved the way for natural classifi cations. At the beginning of the 19th century, de Candolle and Lindley considered a larger number of morphological characters. Since the number of described species became larger, division into sections and subsections was introduced in the genus Rosa. Small diff erences between species and the number of transitional forms lead to taxonomic confusion and created many diff erent classifi cations. Th is problem was not solved in the 20th century either. In addition to the absence of clear diff erences between species, the complexity of the genus is infl uenced by extensive hybridization and incomplete sorting by origin, as well as polyploidy. Diff erent analytical methods used along with traditional, morphological methods help us clarify the phylogenetic relations within the genus and give a clearer picture of the botanical classifi cation of the genus Rosa. Molecular markers are used the most, especially AFLPs and SSRs. Nevertheless, phylogenetic relationships within the genus Rosa have not been fully clarifi ed. Th e diversity of the genus Rosa has not been specifi cally analyzed in Croatia until now. Key words Rosa sp., taxonomy, molecular markers, classifi cation, phylogeny 1 Agricultural School Zagreb, Gjure Prejca 2, 10040 Zagreb, Croatia e-mail: [email protected] 2 University of Zagreb, Faculty of Agriculture, Department of Plant Breeding, Genetics and Biometrics, Svetošimunska cesta 25, 10000 Zagreb, Croatia Received: November , . -

Rosa Canina Linnaeus Common Names: Dog Rose, Dog Brier, Wild Rose (5,6,13)
Rosa canina Linnaeus Common Names: Dog rose, dog brier, wild rose (5,6,13). Etymology: ‘Rosa’ is the Latin word for ‘rose’, and ‘canina’ in Latin means ‘of a dog’ or ‘mean’ (1,3). Botanical synonyms: Rosa corymbifera Borkh., R. dumetorum Thuill., and R. ciliatosepala Blocki (2,6). FAMILY: Rosaceae, the rose family (1) Quick Notable Features: ¬ Alternate, odd-pinnately compound, serrate leaves ¬ Conspicuous stipules, fused to petiole ¬ Showy white/pink flowers with many stamens and pistils in a hypanthium ¬ Bright red hips with no sepals Plant Height: R. canina grows up to 3m tall (10). Subspecies/varieties recognized (6,7): Rosa canina var. dumetorum (Thuill.) Poir., Rosa canina var. canina L., Rosa canina var. corymbifera Rouy, Rosa canina var. andegavensis Arechav., Rosa canina var. evanida (Christ) P.V.Heath, Rosa canina var. frutetorum (Besser) P.V.Heath, Rosa canina var. libertiae (Dumort.) P.V.Heath, Rosa canina var. Montana (Vill.) P.V.Heath, Rosa canina var. sepium Arechav., Rosa canina var. subcanina (Christ) P.V.Heath, Rosa canina subsp. andegavensis (Bastard) Vigo, Rosa canina subsp. virens (Wahlenb.) Šmite. Most Likely Confused with: Rosa eglanteria, R. micrantha, R. setigera, R multiflora, and Rubus ssp. (1,9). Habitat Preference: The species is found in open, disturbed habitats such as roadsides, old pastures, fields, dry banks, and thickets. R. canina requires at least partial sun, and high levels of soil moisture (1,5,9,10). Geographic Distribution in Michigan: The species grows in six counties of the lower peninsula: Benzie, Hillsdale, Kent, Leelanau, Lenawee, and Wayne (2,19). Known Elevational Distribution: In Turkey, R. -

Drought Tolerant Roses
DROUGHT TOLERANT, LOW-MAINTENANCE ROSES ‘Aglaia’ ‘Alberic Barbier’ (Wichuraiana) ‘Albertine’ ‘American Pillar’ (Wichuraiana) ‘Anemone’ (Laevigata hybrid) ‘Banshee’ (Damask) ‘Belinda's Dream’ ‘Belle of Portugal’ ‘Blush Noisette’ ‘Caldwell Pink’ ‘Carefree Beauty’ ‘Cecile Brunner’ ‘Climbing Cecile Brunner’ ‘Climbing Pinkie’ ‘Climbing Souvenir de la Malmaison’ ‘Complicata’ ‘Crepuscule’ ‘Dorothy Perkins’ (Wichuraiana) ‘Double Plum’ ‘Dr. Van Fleet’ ‘Duchesse de Brabant ‘Else Poulsen’ ‘Fortune's Double Yellow’ ‘Francois Juranville’ ‘Frühlingsgold’ (Shrub) ‘Gardenia’ (Wichuraiana) ‘Gloire des Rosomanes’ ‘Golden Chersonese’ (Ecae hybrid) ‘Homestead’ (Hybrid China) ‘John Hopper’ ‘Knock Out’ ‘La Reine’ ‘Lady Hillingdon’ ‘Lawrence Johnston’ (Climber) ‘Marie Daly’ ‘Marie Pavie’ ‘Marie van Houtte’ ‘Mermaid’ ‘Mme. Alfred Carriere’ ‘Mme. Gabriel Luizet’ ‘Mme. Gregoire Staechelin’ ‘Mme. Plantier’ ‘Mrs. Herbert Stevens’ ‘Navaroo Ridge Noisette’ ‘New Dawn’ (Wichuraiana) ‘Papa Gontier’ ‘Paul Ricault’ ‘Paul's Double Musk’ ‘Penelope’ ‘Perle d'Or’ ‘Purezza’ (Banksiae hybrid) ‘Rambling Rector’ ‘Ramona (Laevigata hybrid) ‘Red Dorothy Perkins’ (Wichuraiana) ‘Russelliana’ ‘Sea Foam’ ‘Silver Moon’ wichuraiana ‘Stanwell Perpetual’ (Shrub) ‘Thérèse Bugnet’ (Rugosa hybrid) ‘The Fairy’ ‘Veilchenblau’ ‘Westerland’ ‘White Dorothy’ (Wichuraiana) ‘William Baffin’ ‘Wolley-Dod’ Rosa banksiae (“Lady Banks Rose”) Rosa californica Rosa californica ‘Elsie’ Rosa foetida bicolor (“Austrian Copper”) Rosa foetida persiana (“Persian Yellow”) Rosa gallica Rosa glauca Rosa laevigata (“Cherokee Rose”) Rosa minutifolia Rosa rubiginosa Rosa rugosa cultivars Rosa setigera Rosa spinosissima Rosa wichuraiana Rosa x Harisonii (“Harison's Yellow’”; Shrub) Rosa hugonis Rosa x odorata ‘Mutabilis’ Rosa xanthina (especially. f. hugonis) . -

An Overview of Therapeutic Potentials of Rosa Canina- a Traditionally
WCRJ 2020; 7: e1580 AN OVERVIEW OF THERAPEUTIC POTENTIALS OF ROSA CANINA: A TRADITIONALLY VALUABLE HERB M. KHAZAEI, M.R. KHAZAEI, M. PAZHOUHI Fertility and Infertility Research Center, Health Technology Institute, Kermanshah University of Medical Sciences, Kermanshah, Iran Abstract – Rosa canina L. (Rosacea family) is an ornamental plant with erect prickly shrub and fragrant pink or white flowers, grown for decorative purposes in gardens and landscape designs projects. It is native to Europe, northwest Africa, and western Asia. Its fruits are extensively used worldwide in food preparation. It is traditionally proposed as a dietary supplement and herbal remedy for the prevention and treatment of different human diseases. This review aimed to inves- tigate the pharmacological and therapeutic properties of R. canina in traditional medicine and sci- entific papers. Results from numerous studies indicated that this plant owned many biological po- tencies, including anti-inflammatory, anti-tumor, immunomodulatory, anti-microbial, anti-oxidant, pain reduction, anti-diabetic, anti-hyperlipidemic, neuroprotective, genoprotective, anti-obesity, skin-whitening, and anti-biotic resistance reversal activity as well as exerting a positive influence on the osteoarthritis, anxiety, depression, recognition memory, urinary and reproductive systems disorders, and neutrophil respiratory burst. Nevertheless, the exact mechanism of action for these properties is not fully recognized. Due to the lack of toxicity and side effects, this plant has been considered as a valuable complementary drug for various diseases. Further clinical trials are needed to confirm the reported promising experimental effects in clinical use. KEYWORDS: Rosa canina, Anti-oxidant, Anti-cancer, Anti-diabetes, Osteoarthritis. INTRODUCTION torical background, revealed a wide spectrum of phar- macological potential. -

Safety Assessment of Rosa Canina-Derived Ingredients As Used in Cosmetics
Safety Assessment of Rosa canina-derived Ingredients as Used in Cosmetics Status: Scientific Literature Review for Public Comment Release Date: January 14, 2016 Panel Date: March 31 - April 1, 2016 All interested persons are provided 60 days from the above date to comment on this safety assessment and to identify additional published data that should be included or provide unpublished data which can be made public and included. Information may be submitted without identifying the source or the trade name of the cosmetic product containing the ingredient. All unpublished data submitted to CIR will be discussed in open meetings, will be available at the CIR office for review by any interested party and may be cited in a peer-reviewed scientific journal. Please submit data, comments, or requests to the CIR Director, Dr. Lillian J. Gill. The 2016 Cosmetic Ingredient Review Expert Panel members are: Chair, Wilma F. Bergfeld, M.D., F.A.C.P.; Donald V. Belsito, M.D.; Ronald A. Hill, Ph.D.; Curtis D. Klaassen, Ph.D.; Daniel C. Liebler, Ph.D.; James G. Marks, Jr., M.D.; Ronald C. Shank, Ph.D.; Thomas J. Slaga, Ph.D.; and Paul W. Snyder, D.V.M., Ph.D. The CIR Director is Lillian J. Gill, D.P.A. This report was prepared by Wilbur Johnson, Jr., M.S., Senior Scientific Analyst. © Cosmetic Ingredient Review 1620 L STREET, NW, SUITE 1200 ◊ WASHINGTON, DC 20036-4702 ◊ PH 202.331.0651 ◊ FAX 202.331.0088 ◊ [email protected] INTRODUCTION The safety of the following Rosa canina-derived ingredients as used in cosmetics is reviewed in this safety assessment: -

In Vitro Propagation of Rosa 'Konstancin'
FOLIA HORTICULTURAE Folia Hort. 30(2), 2018, 259-267 Published by the Polish Society DOI: 10.2478/fhort-2018-0022 for Horticultural Science since 1989 ORIGINAL ARTICLE Open access www.foliahort.ogr.ur.krakow.pl In vitro propagation of Rosa ‘Konstancin’ (R. rugosa × R. beggeriana), a plant with high nutritional and pro-health value Agnieszka Wojtania*, Bożena Matysiak Department of Applied Biology Research Institute of Horticulture Konstytucji 3 Maja 1/3, 96-100 Skierniewice, Poland ABSTRACT The aim of the study was to develop an efficient micropropagation system forRosa ‘Konstancin’, an interspecific hybrid between R. rugosa and R. beggeriana, whose fruits have high pro-health value. Shoot cultures were initiated from shoot buds collected in May and August from 15-year-old field-grownRosa ‘Konstancin’ shrubs. The effect and interaction of different concentrations of phytohormones, sucrose and iron sources on in vitro initiation, multiplication and rooting of shoots were studied. The time of collecting explants from donor plants significantly affected the initiation of shoot culture ofRosa ‘Konstancin’. Considerably higher frequency of bud break (100%) was obtained in explants isolated in August as compared to those collected at the end of May (30%). All buds developed into single shoots after 2-4 weeks of growing on the basal Murashige and Skoog medium containing 2.2 µM BAP, 0.3 µM GA3 and 88 mM of sucrose. The highest multiplication rate (4.8 shoots/explant) in a 5-week period was obtained on MS medium containing 50% of nitrogen salts, 3.1 µM BAP, 0.9 µM GA3 and 58 mM sucrose. -
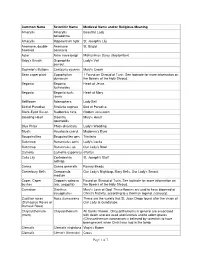
Of 7 Common Name Scientific Name Medieval Name And/Or Religious Meaning Amaryllis Amaryllis Belladonna Beautiful Lady
Common Name Scientific Name Medieval Name and/or Religious Meaning Amaryllis Amaryllis Beautiful Lady belladonna Amaryllis Hippeastrum hybr. St. Joseph's Lily Anemone, double- Anemone St. Brigid flowered coronaria Aster Aster nova-belgii Michaelmas Daisy (September) Baby's Breath Gypsophila Lady's Veil panicul. Bachelor's Buttons Centauria cyannis Mary's Crown Bean caper plant Zygophyllum ? Found on Shroud of Turin. See footnote for more information on dumosum the flowers of the Holy Shroud. Begonia Begonia Heart of Jesus fuchsioides Begonia Begonia fuch. Heart of Mary rosea Bellflower Adenophera Lady Bell Bird of Paradise Streliztia reginae Bird of Paradise Black-Eyed Susan Rudbeckia hirta Golden Jerusalem Bleeding Heart Dicentra Mary's Heart spectabilis Blue Phlox Phlox divaricata Lady's Wedding Bluets Houstonia caerul. Madonna's Eyes Bougainvillea Bougainvillea gen. Trinitaria Buttercup Ranunculus acris Lady's Locks Buttercup Ranunculus sp. Our Lady's Bowl Camelia Camellia (japonica) (Purity) Calla Lily Zantedeshia St. Joseph's Staff aethiop. Canna Canna generalis Rosary Beads Canterbury Bells Campanula Our Lady's Nightcap, Mary Bells, Our Lady's Smock medium Caper, Caper Capparis spinosa Found on Shroud of Turin. See footnote for more information on bushes (var. aegyptia) the flowers of the Holy Shroud. Carnation Dianthus Mary's Love of God. These flowers are said to have bloomed at caryophyllus Christ's Nativity, according to a German legend. (January) Castilian roses Rosa damascena These are the variety that St. Juan Diego found after the vision of (Damascus Roses or Our Lady at Guadalupe. Damask Rose) Chrysanthemum Chrysanthemum All Saints' Flower. Chrysanthemums in general are associated (mum) with death and are used and funerals and to adorn graves (Chrysanthemum coronarium is believed by scientists to have been present when Christ was laid in the tomb. -

Herbal Medicine in Stamps: History of Rosa Canina Through Philately
Galore International Journal of Health Sciences and Research Vol.2; Issue: 4; Oct.-Dec. 2017 Website: www.gijhsr.com Invited Review Paper P-ISSN: 2456-9321 Herbal Medicine in Stamps: History of Rosa Canina through Philately Özüm ERKİN1 1PhD, Research Assistant, Ege University, Faculty of Nursing, Department of Public Health Nursing, 35100, Bornova, İzmir, Turkey ________________________________________________________________________________________________________________ ABSTRACT world's population is treated with natural remedies. [3] Rosa canina, known as "dog The fruit (hips) of R. canina have long been rose" in English and "kuşburnu" in Turkish, recognized for their medicinal and nutritional grows wild in various parts of the world and value. R. canina is native to Europe, produces fruit called rose hips. [2,3] Rose western/central Asia, and northern Africa, and hips have been used in many cultures for rose hips have been used in many cultures for centuries, and are referred to in Tibetan centuries. Recommendations for its use exist in medicine, the works of Avicenna, and even Tibetan medicine, and mentioned in the works [4] of Avicenna, and even in manuscripts of biblical in manuscripts from biblical times. The times. More recently, R. canina hips were used dog rose was a symbol of good luck and in syrup form to prevent scurvy during World strength in the middle Ages. It was believed War II. The importance of R. canina is reflected that burning rose hip incense would ward in philately. Stamps are labels used to show off evil spirits, and houses were ritually payment of postage fees, but their graphic decorated with dog rose blossoms during designs also serve to depict the cultural aspects spring festivals. -

Rosa Multiflora Thun
A WEED REPORT from the book Weed Control in Natural Areas in the Western United States This WEED REPORT does not constitute a formal recommendation. When using herbicides always read the label, and when in doubt consult your farm advisor or county agent. This WEED REPORT is an excerpt from the book Weed Control in Natural Areas in the Western United States and is available wholesale through the UC Weed Research & Information Center (wric.ucdavis.edu) or retail through the Western Society of Weed Science (wsweedscience.org) or the California Invasive Species Council (cal-ipc.org). Rosa eglanteria L.; sweetbriar rose R. eglanteria (=Rosa rubiginosa L. [Jepson Manual 2012]) Rosa canina L.; dog rose Rosa multiflora Thun. ex Murr.; multiflora rose Sweetbriar, dog and multiflora roses Family: Rosaceae Photo courtesy of Richard Old Range: Sweetbriar rose is reported from all western states except Nevada, New Mexico and Arizona. Dog rose is reported in British Columbia, Washington, Idaho, Oregon, Utah and California. Multiflora rose has been reported in British Columbia, Washington, Oregon, California and New Mexico. The only known location in Idaho was eradicated. Habitat: Sweetbriar rose is present in grass and shrublands, while dog rose is found in riparian areas within grasslands and shrublands, within the inland northwestern United States. Multiflora rose occurs in successional fields, pastures, and roadsides. It also may occur in dense forests, particularly near disturbances, such as treefall gaps. R. eglanteria Origin: Sweetbriar and dog roses are both native to Europe, northern Africa and western Asia. Multiflora rose was introduced from Japan, R. multiflora Korea, and eastern China in 1886 as rootstock for ornamental roses.