By Sophie Alden Weaver, BA a Thesis Submitted in Partial Fulfillment Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

English Regional Dialect Lexis in the Names and Occupations of the Gloucestershire Cotswolds: a Reassessment of the Relationship Between Names and Dialects
English regional dialect lexis in the names and occupations of the Gloucestershire Cotswolds: a reassessment of the relationship between names and dialects Item Type Article Authors Parkin, Harry Citation Parkin, H. (2015), ‘English regional dialect lexis in the names and occupations of the Gloucestershire Cotswolds: a reassessment of the relationship between names and dialects’, Dialectologia et Geolinguistica 23, 1, pp. 24–37. DOI 10.1515/dialect-2015-0002 Publisher De Gruyter Journal Dialectologia et Geolinguistica Rights Attribution-NonCommercial-NoDerivatives 4.0 International Download date 24/09/2021 11:02:04 Item License http://creativecommons.org/licenses/by-nc-nd/4.0/ Link to Item http://hdl.handle.net/10034/621769 English regional dialect lexis in the names and occupations of the Gloucestershire Cotswolds: A reassessment of the relationship between names and dialects David Harry Parkin Abstract A number of surname-based studies have presented a relationship between medieval regional dialect lexis and the distribution of associated modern-day surnames. However, by carrying out localised research, it appears that the two might not be so closely linked as previously thought, with discrepancies in the distribution of regionally specific names and equivalent occupational descriptions. As a result, there seems to be a need to reconsider the connection between regional lexicons and corresponding name stocks, which may have been less closely related, at a period of non-hereditary by-naming, than current knowledge suggests. 1. Surnames and dialect It has been widely recognised that the regional distribution of certain by-names and surnames,1 from the medieval period up to the present day, can represent regional distinctions in Middle English (ME) dialect lexis and phonology. -

1 Catherine E. Weaver
Catherine E. Weaver Associate Professor, LBJ School of Public Affairs Distinguished Scholar and Research Coordinator, Robert S. Strauss Center for International Security and Law The University of Texas at Austin 2315 Red River Street, SRH 3.358 Austin, Texas 78712 email: [email protected] Strauss Center main phone: (512) 471-6267 Office Phone: (512) 232-3443 Education Ph.D., Political Science May 2003 University of Wisconsin, Madison BA, Political Science and Russian April 1994 University of Michigan Academic Positions Associate Professor, LBJ School of Public Affairs, September 2010 – present University of Texas at Austin Assistant Professor, LBJ School of Public Affairs, January 2009 – August 2010 University of Texas at Austin Assistant Professor of Political Science August 2002 – December 2008 University of Kansas Research Fellow, Brookings Institution August 2001-2002 Washington, D.C. Publications Books: Catherine Weaver. 2008. Hypocrisy Trap: The World Bank and the Poverty of Reform. Princeton, NJ: Princeton University Press. ~Co-recipient of the Chadwick Alger Prize for best book on International Organizations and Multilateralism, International Studies Association, 2009. (Co-recipient with Benjamin Schiff) ~Winner of the Society of Policy Scientists 2009 Harold D. Lasswell Prize. Catherine Weaver and Nicola Phillips, eds. 2010. International Political Economy and the Transatlantic Divide. London, UK: Routledge Press. 1 Articles and Book Chapters (* = in peer review journal or reviewed as part of book project): *Catherine Weaver and Susan Park. Forthcoming 2012.“The Anatomy of IO Agency: Anticorruption and Environmental Reform at the World Bank.” In Joel Oestreich, ed. Agency in International Organizations. London: Routledge Press. *Catherine Weaver. 2010. "The Politics of Performance Evaluation: Independent Evaluation at the International Monetary Fund" Review of International Organizations, September 2010, pp.365-385. -

Oregon City High School Commencement the Class of 2020
Oregon City High School 135th Annual Commencement The Class of 2020 Sunday, June 7, 2020 Oregon City High School 12:00pm ~ 4:00pm OCSD Board of Education Evon Tekorius, Chair Martha Spiers, Vice Chair Connie Curteman Scott Dahlman Anna Farmer Steve Soll Pamela White District Administration Larry Didway, Superintendent Kyle Laier, Assistant Superintendent Sara Deboy, Director of Teaching and Learning Susan Dodd, Chief Financial Officer John Ogden, Director of Human Resources Todd Nicholson, Director of Special Services Wes Rogers, Bond Manager OCHS Staff The Class of 2020 Nic Liebrecht Lisa Ramage Doug Thomas Maria Elena Margaret Rierson Trecia Thompson Class Motto Lopez-Mabrey Carlene Roley Adam Thygeson Mike Lord Jake Rondema Caralee Thygeson "Life is either a daring adventure, or nothing." John Lucente Brian Ruby Megan Thyken -Helen Keller Amelia Luna Mina Shalada (Gabi) Rush Stephanie Tolke Jane Lundy Amber Salvey Tiffani Traver Kenrik Lynch Karlie Sanchez Susan Trone Senior Class Officers Eric Lynn Linda Schachtel John Tsugawa Em MacDonald Cynthia Schneider Cathy Varner President Annalies Rimes Jeff Marcoe Patty Scribner Craig Vattiat Vice President Zach Finnegan Karen Markowski Cornelia Seigneur Tricia Vermace Secretary-Treasurer Shaylee Cooper Stephanie Mattox Taddesse Sewore Trevor Vollintine Senior Class Rep. Brittan Bratscher Meagan McBride Mark Sexton Julia Voorhies Brent McClain Christopher Sherrick Brian Walters Royce McCullough Svetlana Shevchenko Marcus Washington Pat McDonald Michael Silva Wally Watson Jenny McKenzie Les Sitton Deby Webb ASB Executive Officers Stacey Mills Clarise Sky-Johnson Patty Westphal Linda Montz Carolyn Smith Kris White President Max Reed Alana Morgan Rocky Smith Carey Wilhelm Vice President Zoe Kleiner Kristin Mull Zella Smith Brenda Williams Secretary-Treasurer Grant Didway Tiffany Mutchler Sue Snow Jessica Williams Aaron Newkirk Clinton Springer Tess Wills Alena Norr Kelly Springer Lisa Wilson Robert Osborn Marty Stinson J.J. -
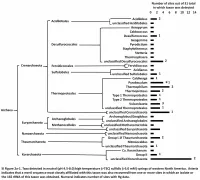
SI Fig 2.Pdf
Figure 2a References Auchtung, T.A., Takacs-Vesbach, C.D. and Cavanaugh, C.A. (2006) 16S rRNA phylogenetic investigation of the candidate division "Korarchaeota". Appl. Environ. Microbiol. 72, 5077- 5082. Barns, S.M., Delwiche, C.F., Palmer, J.D. and Pace, N.R. (1996) Perspectives on archaeal diversity, thermophily and monophyly from environmental rRNA sequences. PNAS (USA) 93, 9188-9193. Barns, S.M., Fundyga, R.E., Jeffries, M.W. and Pace, N.R. (1994) Remarkable archaeal diversity detected in a Yellowstone National Park hot-spring environment. PNAS (USA) 91, 1609-1613. Beam, J.P., Jay, Z.J., Kozubal, M.A. and Inskeep, W.P. (2014) Niche specialization of novel Thaumarchaeota to oxic and hypoxic acidic geothermal springs of Yellowstone National Park. ISME J. 8, 938-951. Connon, S.A., Koski, A.K., Neal, A.L., Wood, S.A. and Magnuson, T.S. (2008) Ecophysiology and geochemistry of microbial arsenic oxidation within a high arsenic, circumneutral hot spring system of the Alvord Desert. FEMS Microbiol. Ecol. 64, 117-128. Dick, J.M. and Shock, E.L. (2013) A metastable equilibrium model for the relative abundances of microbial phyla in a hot spring. Plos One 8, e72395. Dodsworth, J.A., Blainey, P.C., Murugapiran, S.K., Swingley, W.D., Ross, C.A., Tringe, S.G., Chain, P.S.G., Scholz, M.B., Lo, C.-C., Raymond, J., Quake, S.R. and Hedlund, B.P. (2013) Single-cell and metagenomic analyses indicate a fermentative and saccharolytic lifestyle for members of the OP9 lineage. Nature Comm. 4, 1854 doi: 1810.1038/ncomms2884. Dodsworth, J.A. -

Hunterstown Road Site, ) Westinqhouse Electric Corp
UNITED STATES ENVIRONMENTAL PRDTECTION AGENCY REGION III In the Matter of: ) Hunterstown Road Site, ) ) Westinqhouse Electric Corporation, ) Spectra-Kote Corporation ) and Frederick M. Shealer ) ) (Respondents) ) ) PROCEEDING UNDER SECTION 106 (a) ) Docket No. II 1-8 7 -9 -DC Or j.n£ uJpieKfHtNbiVK ENVIRONMENTAL ) RESPONSE, COMPENSATION, AND LIABILITY ) ACT OF 1980 AS AMENDED BY THE ) SUPERFUND AMENDMENTS AND ) REAUTHORIZATION ACT OF 1986, PUB. L. ) NO. 99-499, 100 STAT. 1613(1986). ) (42 U.S.C. <? 9606(a)) ) Consent Agreement and Order The fol lowing Order, agreed to by the United States Environmental Protection Agency ("EPA"), and Westinghouse Electric Corporation, and Spectra-Kote Corporation, and Frederick M. Shealer ("Respondents"), is issued pursuant to the authority vested in the President of the United States of America by Section 106 (a) of the Comprehensive Environmental Response, Compensation, and Liability Act of 1980, as amended by the Superfund Amendments and Reauthorization Act of 1986, Pub. L. No. 99-499 , 100 Stat. 1613 (1986) ("CERCIA"), 42 U.S.C. § 9606(a), and delegated to the Administrator of the EPA on January 23, 1987 by Executive Order 12580, 52 Federal Register 2923, and further delegated to the Regional Admin is :ra tors of EPA. The actions authorized by this Order are consistent with Section 300.65 of the National Oil and Hazardous Substance Contingency Plan ("NCP"), 40 C.F.R. § 300.65. Notice of the issuance of this Order has been given to the Commonwealth of Pennsylvania, The Respondents agree to undertake all terms and conditions of this Consent Agreement and Order and not to contest this Consent Agreement and Order. -

R. Kent Weaver's CV
R. KENT WEAVER Professor of Public Policy and Government, Georgetown University Senior Fellow, Governance Studies Program The Brookings Institution McCourt School of Public Policy Georgetown University Old North 37th and O Streets, N.W. Washington, D.C. 20057 Tel: (202) 687-6953 Fax: (202)687-2289 E-Mail: [email protected] PERSONAL DATA Place of Birth: California, USA Citizenship: U.S.A. EDUCATION Ph.D. Harvard University - Political Science - 1982 M.A. Harvard University - Political Science - 1978 B.A. Haverford College - Political Science - 1975 ACADEMIC POSITIONS HELD 9/02 to present Professor of Public Policy and Government, Georgetown University 9/87 to present Senior Fellow, Governance Studies Program, The Brookings Institution 7/83 to 8/87 Research Associate, Governmental Studies Program, The Brookings Institution 9/81 to 6/83 Instructor, Department of Political Science, The Ohio State University 1 ADMINISTRATIVE POSITIONS HELD 1/04 to 6/06 Associate Dean, Georgetown Public Policy Institute, Georgetown University. 9/00 to 11/02 Co-Director, Welfare Reform and Beyond Initiative, The Brookings Institution VISITING POSITIONS HELD 4/14 to 7/15 Olof Palme Visiting Professor, Department of Political Science, Stockholm University (Funded by the Swedish Research Council) 1/14 to 2/15 Eminent Visiting Professor, Institute of Policy Studies, Universiti Brunei Darussalam 1/11 to 5/11 and Visiting Fellow, European University Institute, Florence 1/12 to 5/12 2/08 to 5/10 Visiting Professor, Masters of Public Affairs Program, Sciences -

Spring 2020 B-M-S
COMMENCEMENT THIS IS NOT AN OFFICIAL GRADUATION LIST While every effort is made to ensure accuracy in this commencement program, printing deadlines may result in omission of some names and use of names of persons not completing graduation requirements as intended. This printed program, therefore, should not be used to determine a student’s academic or degree status. The university’s official registry for conferral of degrees is the student’s permanent academic record as reflected on the student’s transcript, maintained by the Office of the University Registrar. Commencement SPRING 2020 Greetings from the President n behalf of the University of Florida, our faculty and our entire university community, I would like to extend my deepest congratulations to you, the Graduates of 2020. OI celebrate your remarkable accomplishment in earning a degree from one of the world’s leading research institutions. I applaud you for the education you have received, for honing your creative, scientific, or analytical skills and for your achievements as students. I join you in thanking your professors, advisors and family and friends for their contributions to your success. This is a time for celebration, but also for reflection on your future. I am confident that the knowledge and experience you gained as a UF student will serve you well regardless of your career or how you choose to invest your life. The university has benefited from your active engagement and contributions to your classes, programs of study and your peers, and we are grateful for your time here. We hope you will recall these years fondly, and that you will remain connected to UF as active members of our distinguished alumni. -

September 2020
E X E C U T I V E C O U N C I L M E E T I N G MINUTES AND REPORT OF THE INTERNATIONAL EXECUTIVE COUNCIL’S REGULAR MEETING The regular meeting of the International Executive Council was called to order at 11:00 am, by Chairman Erikson, on Tuesday, September 15, 2020, via audio/video conference call. Other members of the council in attendance were Calabro, Calvey, Burgham, Riley, Furco, Wine, Lavin, and Galbraith. INTERNATIONAL PRESIDENT STEPHENSON International President Lonnie R. Stephenson met with the members of the International Executive Council to discuss a variety of matters affecting all branches of the Brotherhood. INTERNATIONAL SECRETARY-TREASURER COOPER International Secretary-Treasurer Kenneth W. Cooper presented financial reports covering the IBEW Pension Fund and the Investment Portfolio of the Brotherhood - both in Canada and in the United States. LEGAL DEFENSE Payments for legal defense, made from the General Fund, were examined and approved in accordance with the requirements of Article X, Section 1, of the IBEW Constitution. FINANCIAL REPORTS The International Secretary-Treasurer's Reports for the various funds of the Brotherhood were presented to the members of the International Executive Council, examined, approved, and filed. APPEALS FILED WITH THE INTERNATIONAL EXECUTIVE COUNCIL The International Executive Council conducted a thorough review of the facts pertaining to an appeal from Local Union 11-member Ivan Bacelic, and it is the decision of the IEC to deny Brother Bacelic’s appeal. ARTICLE XX AND XXI CASES There were no new Article XX or XXI cases during the second quarter of 2019. -

Aes Corporation
THE AES CORPORATION THE AES CORPORATION The global power company A Passion to Serve A Passion A PASSION to SERVE 2000 ANNUAL REPORT ANNUAL REPORT THE AES CORPORATION 1001 North 19th Street 2000 Arlington, Virginia 22209 USA (703) 522-1315 CONTENTS OFFICES 1 AES at a Glance AES CORPORATION AES HORIZONS THINK AES (CORPORATE OFFICE) Richmond, United Kingdom Arlington, Virginia 2 Note from the Chairman 1001 North 19th Street AES OASIS AES TRANSPOWER Arlington, Virginia 22209 Suite 802, 8th Floor #16-05 Six Battery Road 5 Our Annual Letter USA City Tower 2 049909 Singapore Phone: (703) 522-1315 Sheikh Zayed Road Phone: 65-533-0515 17 AES Worldwide Overview Fax: (703) 528-4510 P.O. Box 62843 Fax: 65-535-7287 AES AMERICAS Dubai, United Arab Emirates 33 AES People Arlington, Virginia Phone: 97-14-332-9699 REGISTRAR AND Fax: 97-14-332-6787 TRANSFER AGENT: 83 2000 AES Financial Review AES ANDES FIRST CHICAGO TRUST AES ORIENT Avenida del Libertador COMPANY OF NEW YORK, 26/F. Entertainment Building 602 13th Floor A DIVISION OF EQUISERVE 30 Queen’s Road Central 1001 Capital Federal P.O. Box 2500 Hong Kong Buenos Aires, Argentina Jersey City, New Jersey 07303 Phone: 852-2842-5111 Phone: 54-11-4816-1502 USA Fax: 852-2530-1673 Fax: 54-11-4816-6605 Shareholder Relations AES AURORA AES PACIFIC Phone: (800) 519-3111 100 Pine Street Arlington, Virginia STOCK LISTING: Suite 3300 NYSE Symbol: AES AES ENTERPRISE San Francisco, California 94111 Investor Relations Contact: Arlington, Virginia USA $217 $31 Kenneth R. Woodcock 93% 92% AES ELECTRIC Phone: (415) 395-7899 $1.46* 91% Senior Vice President 89% Burleigh House Fax: (415) 395-7891 88% 1001 North 19th Street $.96* 18 Parkshot $.84* AES SÃO PAULO Arlington, Virginia 22209 Richmond TW9 2RG $21 Av. -

Ltuu Irursru Lit Umily
ltuu irursru lit umily C!!aptaiu !\.lbrrt i;nrrisnn ]tau irustu ]Jnlumt 1 Abraham Pidersen, lJfolenaer lll'rauk .Allabru "ru.rahtgiral <tinmpauu Numbtr ill~rtr 3!1tst Jrnrtu-&rcnnh &trttt, Nrm lnrk Copyright, x 912, by FRANK ALLABEN GENEALOGICAL COMPANY DEDICATION To the Van Deursen Family in general, and, in particular, to my nephew, William Albert Van Deusen, who is pre paring himself to take up this work where I lay it down, I affectionately dedicate these volumes. PREFACE The compiling of this family history was undertaken with the object of preserving for future generations the results of nearly twenty years' gleanings in the field of genealogical research. The work has been done during the odd moments of a very busy life. It had its inception in the endeavor to learn when and from whence came the first of mv name to New Netherland, and then to connect with this parent branch that offshoot which, after the War of the Revolution, settled in Canada,* and of which I am a scion. It has been a fascinating work and full of pleasure; the measure of success which I obtained luring me on and on, encouraged as I was, also, by the assistance of the Reverend Roswell Randall Hoes, Chaplain in the United States Navy, and himself an able genealogist, who pointed out to me where to find rich harvests of data relating to the Dutch in America. In the military field I profited much from the experience of Dr. Charles E. Godfrey in his investigations of Colonial and Revolutionary military data, and I have sought to make my work a valuable book of reference to the family and others on this particular line. -

Benjamin K Tkach
Benjamin K Tkach Department of Political Science and Cell: 903.293.7654 Public Administration [email protected] Mississippi State University [email protected] 105 Bowen Hall www.benjamintkach.com Mississippi State University, MS 39762 Academic Positions Assistant Professor of Political Science, Mississippi State University, 2018- Assistant Professor of Security and Intelligence, King University, 2015-2018 Assistant Research Scientist, Center on Conflict and Development, 2013-2018 USAID Higher Education Solutions Network, Texas A&M University Education Ph.D. Texas A&M University, Political Science, Dec. 2013 B.A., Whitworth University, 2007 Peer Reviewed Publications Tkach, Benjamin. nd. \Private Military and Security Companies, Contract Structure, Market Competition, and Violence in Iraq" Conflict Management and Peace Science, forthcoming. Tkach, Benjamin and Apryl Williams. 2017. \Mobile (In)security? Exploring the Realities of Mobile Phone Use in Conflict Areas" Information, Communication, and Society 21(2):1-16 Fuhrmann, Matthew and Benjamin Tkach. 2015. \Almost Nuclear: Introducing the Nuclear Latency Dataset" Conflict Management and Peace Science 32(4): 443-461. Koch, Michael and Benjamin Tkach. 2012. \Deterring or Mobilizing? The Influence of Government Partisanship and Force on the Frequency, Lethality, and Suicide Attacks of Terror Events"Peace Economics, Peace Science and Public Policy 18(2): 1-27. Peer Reviewed Policy Booklets Tkach, Benjamin. 2017. Special Operations Contacting: 21st Century Approaches for Service and Technology Acquisition. MacDill Air Force Base, Joint Special Operations University Press. (ISBN 978-1-941715-17-8) Kibriya, Shahriar and Benjamin Tkach. 2016. The Effects of School Related Gender-Based Violence on Academic Performance: Evidence from Botswana, Ghana, and South Africa. -

Paul T. Culley Genealogy Papers Finding
A guide to the Paul T. Culley Genealogy Papers Livingston County Historian’s Office 5 Murray Hill Drive Mount Morris, NY 14510 Produced by Holly C. Watson October, 2014 Introduction This collection was arranged and described by Holly C. Watson in order to make the materials more accessible. The papers were organized according to their original arrangement by the researcher and donor, Paul T. Culley, and remain generally alphabetical by surname. Documents in the collection are housed in acid-free file folders, gathered in two (2) acid- free and lignin-free boxes, totaling about .75 cubic feet of records. The documents are mainly handwritten transcriptions of census schedules with scattered family tree charts, correspondence, copies of published genealogies, and original documents. The range of dates in the material is from about 1790 to the 1980s, the bulk being 1810-1925. The two original photograph albums were deconstructed and the photographs labeled and housed in appropriate protective sleeves, the order and any notations being maintained. These photographs have been inventoried to the item level. 2 Guidelines for Use Use of the documents in the collection is for genealogical research and by request and permission of the Livingston County Historian’s Office. Any use of the series material in publications must be cited as follows, “Courtesy of Livingston County Historian’s Office, New York”. Records can be viewed Monday through Friday from 1:00 PM to 4:30 PM at the County Historian’s Office. To arrange to conduct research with the documents in the collection, contact Amie Alden, Livingston County Historian by mail at: 5 Murray Hill Drive, Mount Morris, NY, by phone at (585) 243-7955 or (585) 335-1730 or by e-mail at [email protected].