Pregnenolone Can Protect the Brain from Cannabis Intoxication
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pregnenolone Introduced 1997
Product Information Sheet – September 2016 Pregnenolone Introduced 1997 What Is It? • This product should not be taken by individuals with healthy levels of pregnenolone. Pregnenolone, 3-alpha-hydroxy-5-beta-pregnen-20-one, is a precursor to over 150 steroid hormones and is produced naturally in • Pregnenolone is best utilized by individuals over 40, and should the body from cholesterol. not be used to enhance athletic ability or endurance. Uses For Pregnenolone Are There Any Precautions Or Potential Side Memory Support: Animal studies have reported that pregnenolone Effects? may help to enhance memory by modulating N-methyl-D-aspartate Precautions: (NMDA) and gamma aminobutyrate (GABA) receptor activity in the brain. One study suggested that pregnenolone helped promote • NOT FOR USE BY INDIVIDUALS UNDER THE AGE OF 18. post-training task learning and memory.* • DO NOT USE IF PREGNANT OR NURSING. Immune Health: One study indicated that the 7-hydroxy metabolites • KEEP OUT OF REACH OF CHILDREN. from pregnenolone may help promote healthy immune system • Consult a physician or licensed qualified health professional before response.* using this product if you have, or have a family history of breast Mood Support: Pregnenolone has been reported to help promote cancer, prostate cancer, prostate enlargement, heart disease, low feelings of emotional well-being. One study suggested that “good” cholesterol (HDL), or if you are using any other dietary pregnenolone supported positive mood and feelings of motivation supplement, prescription drug, or over-the-counter drug. by mediating dopamine release.* • Do not exceed the recommended serving. Exceeding the What Is The Source? recommended serving may cause serious adverse health effects. -

Comparison of Pregnenolone Sulfate, Pregnanolone and Estradiol Levels
Rustichelli et al. The Journal of Headache and Pain (2021) 22:13 The Journal of Headache https://doi.org/10.1186/s10194-021-01231-9 and Pain SHORT REPORT Open Access Comparison of pregnenolone sulfate, pregnanolone and estradiol levels between patients with menstrually-related migraine and controls: an exploratory study Cecilia Rustichelli1, Elisa Bellei2, Stefania Bergamini2, Emanuela Monari2, Flavia Lo Castro3, Carlo Baraldi4, Aldo Tomasi2 and Anna Ferrari4* Abstract Background: Neurosteroids affect the balance between neuroexcitation and neuroinhibition but have been little studied in migraine. We compared the serum levels of pregnenolone sulfate, pregnanolone and estradiol in women with menstrually-related migraine and controls and analysed if a correlation existed between the levels of the three hormones and history of migraine and age. Methods: Thirty women (mean age ± SD: 33.5 ± 7.1) with menstrually-related migraine (MM group) and 30 aged- matched controls (mean age ± SD: 30.9 ± 7.9) participated in the exploratory study. Pregnenolone sulfate and pregnanolone serum levels were analysed by liquid chromatography-tandem mass spectrometry, while estradiol levels by enzyme-linked immunosorbent assay. Results: Serum levels of pregnenolone sulfate and pregnanolone were significantly lower in the MM group than in controls (pregnenolone sulfate: P = 0.0328; pregnanolone: P = 0.0271, Student’s t-test), while estradiol levels were similar. In MM group, pregnenolone sulfate serum levels were negatively correlated with history of migraine (R2 = 0.1369; P = 0.0482) and age (R2 = 0.2826, P = 0.0025) while pregnenolone sulfate levels were not age-related in the control group (R2 = 0.04436, P = 0.4337, linear regression analysis). -

Bulk Drug Substances Nominated for Use in Compounding Under Section 503B of the Federal Food, Drug, and Cosmetic Act
Updated June 07, 2021 Bulk Drug Substances Nominated for Use in Compounding Under Section 503B of the Federal Food, Drug, and Cosmetic Act Three categories of bulk drug substances: • Category 1: Bulk Drug Substances Under Evaluation • Category 2: Bulk Drug Substances that Raise Significant Safety Risks • Category 3: Bulk Drug Substances Nominated Without Adequate Support Updates to Categories of Substances Nominated for the 503B Bulk Drug Substances List1 • Add the following entry to category 2 due to serious safety concerns of mutagenicity, cytotoxicity, and possible carcinogenicity when quinacrine hydrochloride is used for intrauterine administration for non- surgical female sterilization: 2,3 o Quinacrine Hydrochloride for intrauterine administration • Revision to category 1 for clarity: o Modify the entry for “Quinacrine Hydrochloride” to “Quinacrine Hydrochloride (except for intrauterine administration).” • Revision to category 1 to correct a substance name error: o Correct the error in the substance name “DHEA (dehydroepiandosterone)” to “DHEA (dehydroepiandrosterone).” 1 For the purposes of the substance names in the categories, hydrated forms of the substance are included in the scope of the substance name. 2 Quinacrine HCl was previously reviewed in 2016 as part of FDA’s consideration of this bulk drug substance for inclusion on the 503A Bulks List. As part of this review, the Division of Bone, Reproductive and Urologic Products (DBRUP), now the Division of Urology, Obstetrics and Gynecology (DUOG), evaluated the nomination of quinacrine for intrauterine administration for non-surgical female sterilization and recommended that quinacrine should not be included on the 503A Bulks List for this use. This recommendation was based on the lack of information on efficacy comparable to other available methods of female sterilization and serious safety concerns of mutagenicity, cytotoxicity and possible carcinogenicity in use of quinacrine for this indication and route of administration. -

Pregnenolone, a Fruit of Cholesterol
PREGNENOLONE, A FRUIT OF CHOLESTEROL Mother of Progesterone & D.H.E.A. The following information comes from Dr. Ray Peat, who has done pioneering research on the anti-aging steroids, pregnenolone, progesterone and DHEA (Dehydroepiandrosterone). I have included excerpts from his writings plus the results of interviews. Research references are provided when available, but in many cases, I could only describe the group of researchers who did the experiment. My purpose for this article is not to start a riot but to illustrate why I think it’s dangerous to artificially inhibit cholesterol formation in your body with drugs and synthetic foods. Dr. Peat accidentally discovered the effects of pregnenolone when he took some vitamin E containing a residue of pregnenolone that was left over from an experiment in solubility. Peat had been suffering from a variety of complaints, including " inflammation of the arteries, dental abscesses, asthma, migraines, and colitis ." When he took the vitamin E containing some pregnenolone in the vitamin E, he crawled out of his sick bed, took a pinch of pure pregnenolone and felt immediately better. All of his symptoms gradually disappeared and in ten weeks, his appearance changed. Many aging characteristics, such as sagging skin, "chicken neck," bags under the eyes, etc. receded. These changes were dramatically reported in a passport photo, taken one year before pregnenolone and another one taken 10 weeks after pregnenolone therapy was initiated. I regret that the original photos are not available for inclusion here. When I saw this photo, presented in one of his newsletters, I fell off my chair, dashed to the phone and called Dr. -

Neurosteroids: Pregnenolone in Human Sciatic Nerves
Proc. Natl. Acad. Sci. USA Vol. 89, pp. 6790-6793, August 1992 Neurobiology Neurosteroids: Pregnenolone in human sciatic nerves (dehydroepiandrosterone/mass spectrometry/steroid sulfates/steroid fatty acid esters) ROBERT MORFIN*t, JACQUES YOUNG*, COLETTE CORPtCHOT*, BORJE EGESTADt, JAN SJOVALLt, AND ETIENNE-EMILE BAULIEU*§ *Institut National de la Santd et de la Recherche MWdicale U33, Laboratoire Hormones, 94276 Bicetre Cedex, France; and tDepartment of Physiological Chemistry, Karolinska Institutet, Stockholm, Sweden Contributed by Etienne-Emile Baulieu, March 30, 1992 ABSTRACT The characterization and quantification of Tissues. Tissues were obtained <48 hr after death from pregnenolone in human sciatic nerves were undertaken, fol- cadavers of both sexes bequeathed to medical research. lowing previous demonstration of the synthesis of this steroid Corpses were kept refrigerated at 40C. Ages ranged from 44 in rat brain oligodendrocytes, to explore the hypothesis that to 90 years. Causes of death were not recorded. Portions of Schwann cells may demonstrate the same biosynthetic activity. sciatic nerves, tendons, and muscle were taken close to the Pregnenolone was definitively identified by mass spectrometry knee. Samples of spleen were obtained in one case. The and quantified by specific radioinmunoassay. Its concentra- collected samples (0.675-4.870 g) were first weighed and then tion (mean ± SD, 63.9 ± 45.9 ng/g of wet tissue, n = 12) was frozen in liquid nitrogen until processed further. 2100 times the plasma level and concentration found in Extraction and Purification Procedures. Frozen samples in tendons and muscle. No correlation was found with sex or age. liquid nitrogen were first pulverized in a mortar and then Free dehydroepiandrosterone as well as sulfate and fatty acid homogenized in phosphate-buffered saline at 40C. -
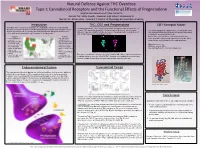
Introduction Endocannabinoid System Cannabinoid Tetrad THC, CB1 And
Natural Defense Against THC Overdose Type-1 Cannabinoid Receptors and the Functional Effects of Pregnenolone Brigitte Rios Llamosa and Alexis Camacho Advisor: Mr. Justin Spaeth, Messmer High School, Milwaukee WI Mentor: Dr. Aaron Miller - Assistant Professor of Physiology at Concordia University Introduction THC, CB1 and Pregnenolone CB1 Receptor Model Cannabis, often referred to as marijuana, is a drug produced from the Cannabis plant. Tetrahydrocannabinol (THC), the active ingredient in marijuana, also activates the CB1 Recently, marijuana has become an often debated topic as people work to legalize its use receptor. THC and similar drugs have therapeutic potential in the treatment of pain, Our model highlights the amino acids E133 and R409, which for both recreational (as in Colorado) and medical purposes. Marijuana is able to help Alzheimer’s disease, anxiety, arthritis, and cancer. A downside to the medicinal use of form hydrogen bonds with pregnenolone and are required for relieve pain, but it can also lower performance in everyday tasks. THC is that it also induces psychotropic effects. its binding to the allosteric site of CB1. Negatives Positives Both of these amino acids are colored in cpk color and • Poor coordination of • Can help control connected with a strut to our pregnenolone molecule. Other movement epileptic seizures areas that are highlighted are color-coded as follows: • Afterwards, users feel • May decrease anxiety tired or depressed • Can slow progression Alpha helixes are red. • Increases heartbeat and of diseases such as risk of heart attack Alzheimer’s disease Struts are colored white. • Inability to understand • Has been used to treat Non-motif portions are colored cornflower blue. -

Michigan Medicaid Program Maximum Allowable Cost (MAC) Pricing
1 ** Confidential and Proprietary ** For Week Ending 09/22/2021 Michigan Medicaid Program Maximum Allowable Cost (MAC) Pricing Due to frequent changes in price and product availability, this listing should NOT be considered all-inclusive. Updated listings will be posted weekly. Effective MAC Generic Name Strength Form Route Date Price 0.9 % SODIUM CHLORIDE 0.9 % VIAL INJECTION 01/27/2021 0.07271 0.9 % SODIUM CHLORIDE 0.9 % IV SOLN INTRAVEN 03/17/2021 0.00327 1,2-PENTANEDIOL 100 % LIQUID MISCELL 10/28/2020 0.56682 2-DEOXY-D-GLUCOSE POWDER MISCELL 12/09/2020 54.63250 5-HYDROXYTRYPTOPHAN (5-HTP) 100 MG CAPSULE ORAL 01/20/2021 0.33500 5HYDROXYTRYPTOPHAN(OXITRIPTAN) POWDER MISCELL 12/16/2020 11.24640 7-OXODEHYDROEPIANDROSTERONE,MC 100 % POWDER MISCELL 10/07/2020 7.36092 ABACAVIR SULFATE 20 MG/ML SOLUTION ORAL 12/30/2020 0.67190 ABACAVIR SULFATE 300 MG TABLET ORAL 06/09/2021 0.89065 ABACAVIR SULFATE/LAMIVUDINE 600-300MG TABLET ORAL 03/10/2021 2.11005 ABACAVIR/LAMIVUDINE/ZIDOVUDINE 150-300 MG TABLET ORAL 12/11/2019 26.74276 ABIRATERONE ACETATE 500 MG TABLET ORAL 09/20/2021 145.40750 ACACIA POWDER MISCELL 01/22/2020 0.14149 ACAI BERRY EXTRACT 500 MG CAPSULE ORAL 05/01/2019 0.06689 ACAMPROSATE CALCIUM 333 MG TABLET DR ORAL 07/29/2020 0.97128 ACARBOSE 100 MG TABLET ORAL 09/08/2021 0.40079 ACARBOSE 50 MG TABLET ORAL 11/11/2020 0.28140 ACARBOSE 25 MG TABLET ORAL 01/13/2021 0.22780 ACEBUTOLOL HCL 200 MG CAPSULE ORAL 09/08/2021 0.88574 ACEBUTOLOL HCL 400 MG CAPSULE ORAL 03/31/2021 1.02443 ACESULFAME POTASSIUM 100 % POWDER MISCELL 12/09/2020 3.34620 MI MAC Pricing Information contained in this document is confidential and proprietary and is available to you solely for the purpose of assisting with claim processing and program reimbursement analysis. -

Memory-Enhancing Effects in Male Mice of Pregnenolone and Steroids
Proc. Nati. Acad. Sci. USA Vol. 89, pp. 1567-1571, March 1992 Neurobiology Memory-enhancing effects in male mice of pregnenolone and steroids metabolically derived from it (memory enhancement/pregnenolone sulfate/receptors/immediate-early genes/aging) JAMES F. FLOOD*t, JOHN E. MORLEY*, AND EUGENE ROBERTSt§ *Genatnc Research Education and Clinical Center, Veterans Administration Medical Center, St. Louis, MO 63106, and St. Louis University School of Medicine, Division of Geriatric Medicine, St. Louis, MO 63104; and tDepartment of Neurobiochemistry, Beckman Research Institute of the City of Hope, Duarte, CA 91010 Contributed by Eugene Roberts, November 8, 1991 ABSTRACT Immediate post-training intracerebroven- postmitotic differentiated states was favored in both neurons tricular administration to male mice of pregnenolone (P), and glia (7, 8). pregnenolone sulfate (PS), dehydroepiandrosterone (DHEA), The water-soluble DHEAS, administered intracerebro- dehydroepiandrosterone sulfate (DHEAS), androstenedione, ventricularly (i.c.v.) or subcutaneously (s.c.) after training, testosterone, dihydrotestosterone, or aldosterone caused im- showed convincing memory-enhancing (ME) effects in foot- provement ofretention for footshock active avoidance training, shock active avoidance training (FAAT) in undertrained male while estrone, estradiol, progesterone, or 16.8-bromoepi- mice. DHEAS administered i.c.v. facilitated retention for androsterone did not. Dose-response curves were obtained for step-down passive avoidance training. Retention of FAAT P, PS, DHEA, and testosterone. P and PS were the most potent, was improved when DHEAS was given in taste-camouflaged PS showing significant effects at 3.5 fmol per mouse. The active drinking water for 1 week before and 1 week after training, steroids did not show discernible structural features or known while DHEAS in the drinking water for 2 weeks did not membrane or biochemical effects that correlated with their improve acquisition (9). -

Downloaded from Bioscientifica.Com at 10/01/2021 09:36:04PM Via Free Access 68 R KANCHEVA and Others
67 Relationships of circulating pregnanolone isomers and their polar conjugates to the status of sex, menstrual cycle, and pregnancy Radmila Kancheva, Martin Hill, David Cibula1, Helena Vcˇela´kova´, Lyudmila Kancheva, Jana Vrbı´kova´, Toma´sˇ Fait1, Antonı´nParˇ´ızek1 and Luboslav Sta´rka Institute of Endocrinology, Na´rodnı´trˇı´da 8, CZ 116 94 Prague 1, Czech Republic 1Department of Gynecology and Obstetrics, 1st Medical Faculty, Charles University, Apolina´rˇska´ 18, CZ 128 51 Prague 2, Czech Republic (Requests for offprints should be addressed to M Hill; Email: [email protected]) Abstract Pregnanolone isomers (PIs) and their polar conjugates (PICs) found in P indicate the more intensive conjugation of 5a-PI modulate ionotropic receptors such as g-aminobutyric acid during pregnancy. This mechanism probably provides for the or pregnane X receptors. Besides, brain synthesis, PI elimination of neuroinhibitory P3a5a in the maternal penetrates the blood–brain barrier. We evaluated the compartment. Additionally, our result points to a limited physiological importance of PI respecting the status of sex, sulfation capacity for neuroinhibitory P3a5b in P. In contrast menstrual cycle, and pregnancy. Accordingly, circulating to the situation in M, F, and L where the P3a5bC is the most levels of allopregnanolone (P3a5a), isopregnanolone abundant PIC, and P3a5b is present in minor quantities (P3b5a), pregnanolone (P3a5b), epipregnanolone (P3b5b), compared with the P3a5a,P3a5b may acquire physiological their polar conjugates, and related steroids were measured in importance during pregnancy, contributing to the sustaining 15 men (M), 15 women in the follicular phase (F), 16 women thereof. On the other hand, the declining formation of in the luteal phase (L), and 30 women in the 36th week of P3a5b may participate in the initiation of parturition, given gestation (P) using GC–MS. -

The Importance of Being Dehydroepiandrosterone Sulfate
Biochemical Pharmacology, Vol. 57, pp. 329–346, 1999. ISSN 0006-2952/99/$–see front matter © 1999 Elsevier Science Inc. All rights reserved. PII S0006-2952(98)00246-9 COMMENTARY The Importance of Being Dehydroepiandrosterone Sulfate (in the Blood of Primates) A LONGER AND HEALTHIER LIFE? Eugene Roberts* DEPARTMENT OF NEUROBIOCHEMISTRY,BECKMAN RESEARCH INSTITUTE OF THE CITY OF HOPE, DUARTE, CA 91010, U.S.A. ABSTRACT. The general aging sequence in tissues of healthy human beings is proposed to be: capillary endothelial cell damage 3 arteriosclerosis 3 decreased blood flow 3 metabolic dysregulation 3 secondary a tissue damage. Molecular O2 is an obligatory substrate for the successive syntheses of 17 -OH pregnenolone and dehydroepiandrosterone (DHEA) by cytochrome P450c17 in the zona reticularis of the adrenal cortex, in which 3 3 3 it is suggested that arteriosclerosis decreased blood flow O2 and glucose deficit decreased O2-requiring synthesis of DHEA 3 eventual decrease in number of DHEA-synthesizing cells. Aging changes in the zona reticularis synergize with those in the hypothalamo-hypophyseal machinery that controls it neurally and hormonally, with ACTH-evoked pulsatile floods of cortisol coming from the adrenal zona fasciculata, with the onslaught of free radicals generated by the metabolism of catecholamines released from interdigitating cells of the adrenal medulla, and with age-correlated disabilities of erythrocytes to bind and release O2 to decrease the viability of the DHEA and dehydroepiandrosterone sulfate (DHEAS)-forming cells. One of the chief functions of serum DHEAS in the male may be to act as an allosteric facilitator of the binding of testosterone (T) to serum albumin, thereby helping target T to specific receptors and to allosteric sites for rapid and efficient action at the cellular level. -

The Role of Pregnenolone Sulphate in Spatial Orientation-Acquisition
Behavioural Processes 99 (2013) 130–137 Contents lists available at ScienceDirect Behavioural Processes journal homepage: www.elsevier.com/locate/behavproc The role of pregnenolone sulphate in spatial orientation-acquisition and retention: An interplay between cognitive potentiation and mood regulation ∗ Fulvio Plescia, Rosa A.M. Marino, Emanuele Cannizzaro, Anna Brancato, Carla Cannizzaro Department of Sciences for Health Promotion and Mother and Child Care “Giuseppe D’Alessandro”, University of Palermo, V. Vespro 129, 90127 Palermo, Italy a r t i c l e i n f o a b s t r a c t Article history: Neurosteroids can alter neuronal excitability interacting with specific neurotransmitter receptors, thus Received 23 May 2013 affecting several functions such as cognition and emotionality. In this study, we investigated, in adult Received in revised form 4 July 2013 male rats, the effects of the acute administration of pregnenolone-sulfate (PREGS) (10 mg/Kg, s. c.) on Accepted 5 July 2013 cognitive processes using the Can test, a non aversive spatial/visual task which allows the assessment of spatial information-acquisition during the baseline training, and of memory retention in the longitudi- Keywords: nal study. Furthermore, on the basis of PREGS pharmacological profile, the modulation of depressive-like Pregnenolone-sulphate behaviour was also evaluated in the forced swim test (FST). Our results indicate that acute PREGS induces: Spatial orientation-acquisition an improvement in spatial orientation-acquisition and in reference memory, during the baseline training; Spatial orientation-retention a strengthening effect on reference and working memory during the longitudinal study. A decrease in Cognitive map Depressive-like behaviour immobility time in the FST has also been recorded. -

Pregnenolone
ENDOCRINE HEALTH Pregnenolone CLINICAL APPLICATIONS • Promotes Normal Hormonal Balance • Helps Maintain Healthy Memory and Cognitive Function • Supports Mood Regulation • Supports the Stress Response System by Acting as Hormonal Precursor • Supports Learning and Memory What is Pregnenolone? of other vital neurosteroids such as DHEA,1 declining levels of Pregnenolone plays a key role in hormonal balance as a key pregnenolone could leave brain cells increasingly vulnerable to precursor to cortisol, dehydroepiandrosterone (DHEA) and overstimulation by neurotransmitters like glutamate,1,2 thereby progesterone, and helps to maintain balance in the body’s affecting mood and cognition. stress response system. In addition, pregnenolone has been shown to support a balanced mood and promote cognitive Mood Regulation† health by modulating the transmission of messages between Research has shown pregnenolone to be beneficial for mood neurons, influencing learning and memory processes. Since support and balance.3 Specifically, pregnenolone is reported there is strong evidence that pregnenolone levels diminish to have a positive effect on neuronal excitability and synaptic with advancing age, restoring these levels may also help plasticity, and has many other functions associated with mood support overall brain function and sense of well-being. Each regulation, neuroprotection from free radicals, balancing the serving includes 10 mg of pregnenolone in a scored, quick- stress response and improving cognitive performance.3 dissolved tablet, allowing for incremental dosing as needed. In a study of 15 adults with mood imbalance, blood levels Overview of pregnenolone were lower among those with low mood, Pregnenolone is a prohormone that is synthesized in the compared to controls.4 Among 70 adults with mood brain and adrenals, but also in the liver, skin, brain, testicles, imbalance who received either pregnenolone or placebo, the ovaries, and retina.