Evaluation of Treatment by Pulsed Electromagnetic Fields in a Rabbit Hyphema Model
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Summary Benchmarks for Preferred Practice Pattern® Guidelines
SUMMARY BENCHMARKS FOR PREFERRED PRACTICE PATTERN® GUIDELINES TABLE OF CONTENTS Summary Benchmarks for Preferred Practice Pattern Guidelines Introduction . 1 Glaucoma Primary Open-Angle Glaucoma (Initial Evaluation) . 3 Primary Open-Angle Glaucoma (Follow-up Evaluation) . 5 Primary Open-Angle Glaucoma Suspect (Initial and Follow-up Evaluation) . 6 Primary Angle-Closure Disease (Initial Evaluation and Therapy) . 8 Retina Age-Related Macular Degeneration (Initial and Follow-up Evaluation) . 10 Age-Related Macular Degeneration (Management Recommendations) . 11 Diabetic Retinopathy (Initial and Follow-up Evaluation) . 12 Diabetic Retinopathy (Management Recommendations) . 13 Idiopathic Epiretinal Membrane and Vitreomacular Traction (Initial Evaluation and Therapy) . 14 Idiopathic Macular Hole (Initial Evaluation and Therapy) . 15 Posterior Vitreous Detachment, Retinal Breaks, and Lattice Degeneration (Initial and Follow-up Evaluation) . 17 Retinal and Ophthalmic Artery Occlusions (Initial Evaluation and Therapy) . 18 Retinal Vein Occlusions (Initial Evaluation and Therapy) . 19 Cataract/Anterior Segment Cataract (Initial and Follow-up Evaluation) . 20 Cornea/External Disease Bacterial Keratitis (Initial Evaluation) . 22 Bacterial Keratitis (Management Recommendations) . 23 Blepharitis (Initial and Follow-up Evaluation) . 24 Conjunctivitis (Initial Evaluation) . 25 Conjunctivitis (Management Recommendations) . 26 Corneal Ectasia (Initial Evaluation and Follow-up) . 27 Corneal Edema and Opacification (Initial Evaluation) . 28 Corneal Edema -
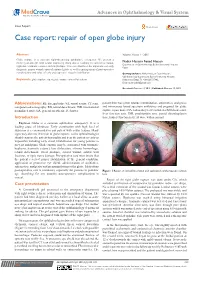
Repair of Open Globe Injury
Advances in Ophthalmology & Visual System Case Report Open Access Case report: repair of open globe injury Abstract Volume 3 Issue 1 - 2015 Globe rupture is a common sight-threatening ophthalmic emergency. We present a twelve-year-old girl with ocular trauma by sharp object resulting in corneal laceration, Nader Hussein Fouad Hassan Department of Ophthalmology, Benha University Hospital, hyphema, traumatic cataract and iris prolapse. This case illustrates the importance of early Egypt diagnosis, proper wound repair of ruptured globe as well as management of post-operative complications and value of early post-operative visual rehabilitation. Correspondence: Nader Hussein Fouad Hassan, Ophthalmology department, Benha University Hospital, Keywords: globe rupture, open globe injury, corneal laceration Kaliyobeya, Egypt, Tel +201224727982, Email Received: October 16, 2015 | Published: October 19, 2015 Abbreviations: FB, foreign body; VA, visual acuity; CT scan, patient then was given tetanus immunization, antiemetics, analgesics computerized tomography; RD, retinal detachment; INR, international and intravenous broad spectrum antibiotics and prepared for globe normalized ratio; GA, general anesthesia; D, diopter rupture repair under GA. Laboratory tests included a full blood count, liver function tests, INR, prothrombin time, partial thromboplastin Introduction time, kidney function tests, all were within normal. Ruptured Globe is a common ophthalmic emergency. It is a leading cause of blindness. Early examination with high level of suspicion is recommended in any patient with ocular trauma. Many signs may obscure detection of globe rupture, so the ophthalmologist should examine the patient thoroughly and treat the patient as early as impossible including early visual rehabilitation for young patients to prevent amblyopia. Globe rupture may be associated with traumatic hyphema, traumatic cataract, lens dislocation, vitreous hemorrhage, retinal detachment, uveal prolapse, scleral wound, orbital wall fractures, or optic nerve damage. -

URGENT/EMERGENT When to Refer Financial Disclosure
URGENT/EMERGENT When to Refer Financial Disclosure Speaker, Amy Eston, M.D. has a financial interest/agreement or affiliation with Lansing Ophthalmology, where she is employed as a ophthalmologist. 58 yr old WF with 6 month history of decreased vision left eye. Ache behind the left eye for 2-3 months. Using husband’s contact lens solution made it feel better. Seen by two eye care professionals. Given glasses & told eye exam was normal. No past ocular history Medical history of depression Takes only aspirin and vitamins 20/20 OD 20/30 OS Eye Pressure 15 OD 16 OS – normal Dilated fundus exam & slit lamp were normal Pupillary exam was normal Extraocular movements were full Confrontation visual fields were full No red desaturation Color vision was slightly decreased but the same in both eyes Amsler grid testing was normal OCT disc – OD normal OS slight decreased RNFL OCT of the macula was normal Most common diagnoses: Dry Eye Optic Neuritis Treatment - copious amount of artificial tears. Return to recheck refraction Visual field testing Visual Field testing - Small defect in the right eye Large nasal defect in the left eye Visual Field - Right Hemianopsia. MRI which showed a subacute parietal and occipital lobe infarct. ANISOCORIA Size of the Pupil Constrictor muscles innervated by the Parasympathetic system & Dilating muscles innervated by the Sympathetic system The Sympathetic System Begins in the hypothalamus, travels through the brainstem. Then through the upper chest, up through the neck and to the eye. The Sympathetic System innervates Mueller’s muscle which helps to elevate the upper eyelid. -

Differentiate Red Eye Disorders
Introduction DIFFERENTIATE RED EYE DISORDERS • Needs immediate treatment • Needs treatment within a few days • Does not require treatment Introduction SUBJECTIVE EYE COMPLAINTS • Decreased vision • Pain • Redness Characterize the complaint through history and exam. Introduction TYPES OF RED EYE DISORDERS • Mechanical trauma • Chemical trauma • Inflammation/infection Introduction ETIOLOGIES OF RED EYE 1. Chemical injury 2. Angle-closure glaucoma 3. Ocular foreign body 4. Corneal abrasion 5. Uveitis 6. Conjunctivitis 7. Ocular surface disease 8. Subconjunctival hemorrhage Evaluation RED EYE: POSSIBLE CAUSES • Trauma • Chemicals • Infection • Allergy • Systemic conditions Evaluation RED EYE: CAUSE AND EFFECT Symptom Cause Itching Allergy Burning Lid disorders, dry eye Foreign body sensation Foreign body, corneal abrasion Localized lid tenderness Hordeolum, chalazion Evaluation RED EYE: CAUSE AND EFFECT (Continued) Symptom Cause Deep, intense pain Corneal abrasions, scleritis, iritis, acute glaucoma, sinusitis, etc. Photophobia Corneal abrasions, iritis, acute glaucoma Halo vision Corneal edema (acute glaucoma, uveitis) Evaluation Equipment needed to evaluate red eye Evaluation Refer red eye with vision loss to ophthalmologist for evaluation Evaluation RED EYE DISORDERS: AN ANATOMIC APPROACH • Face • Adnexa – Orbital area – Lids – Ocular movements • Globe – Conjunctiva, sclera – Anterior chamber (using slit lamp if possible) – Intraocular pressure Disorders of the Ocular Adnexa Disorders of the Ocular Adnexa Hordeolum Disorders of the Ocular -

A Case of Uveitis-Hyphema-Glaucoma Syndrome Due to Express Miniature Implantation
Henry Ford Health System Henry Ford Health System Scholarly Commons Case Reports Medical Education Research Forum 2020 5-2020 A Case of Uveitis-Hyphema-Glaucoma Syndrome due to ExPRESS Miniature Implantation Andrew Hou Henry Ford Health System, [email protected] Madeline Hasbrook Henry Ford Health System David A. Crandall Henry Ford Health System, [email protected] Follow this and additional works at: https://scholarlycommons.henryford.com/merf2020caserpt Recommended Citation Hou, Andrew; Hasbrook, Madeline; and Crandall, David A., "A Case of Uveitis-Hyphema-Glaucoma Syndrome due to ExPRESS Miniature Implantation" (2020). Case Reports. 135. https://scholarlycommons.henryford.com/merf2020caserpt/135 This Poster is brought to you for free and open access by the Medical Education Research Forum 2020 at Henry Ford Health System Scholarly Commons. It has been accepted for inclusion in Case Reports by an authorized administrator of Henry Ford Health System Scholarly Commons. A Case of Uveitis-Hyphema-Glaucoma Syndrome due to ExPRESS Miniature Implantation Andrew Hou MD, Madeline Hasbrook MD, David Crandall MD Department of Ophthalmology, Henry Ford Health System, Detroit, Michigan Purpose Results Discussion To report a case of a 69-year-old patient We believe etiology for the UGH who developed uveitis-glaucoma- syndrome the significant iridodonesis seen hyphema (UGH) syndrome after an on gonioscopy. In the interim between uneventful ExPRESS (Alcon, Fort Worth, surgery and presentation, the patient had TX) mini shunt surgery for advanced been prescribed tamsulosin for his benign primary open-angle glaucoma. To our prostatic hyperplasia. knowledge, this is the first case report Tamsulosin has been well established as describing glaucoma device induced UGH the cause of intraoperative floppy iris syndrome and its successful treatment syndrome secondary to iris dilator atrophy. -

Two Cases of Endogenous Endophthalmitis That Progressed To
rine to more distal vessels may have led to vasoconstric- References tion and subsequent vasospasm. In conclusion, epinephrine can lead to OAO following 1. Niemi G. Advantages and disadvantages of adrenaline in accidental intra-arterial injection of subcutaneously ad- regional anaesthesia. Best Pract Res Clin Anaesthesiol ministered local anesthetics. Hence, physicians should 2005;19:229-45. carefully administer local anesthesia while considering the 2. Park KH, Kim YK, Woo SJ, et al. Iatrogenic occlusion of possibility that such a complication may occur. the ophthalmic artery after cosmetic facial filler injections: a national survey by the Korean Retina Society. JAMA Byung Gil Moon Ophthalmol 2014;132:714-23. Retina Center, Department of Ophthalmology, HanGil Eye 3. Lazzeri D, Agostini T, Figus M, et al. Blindness following Hospital, Incheon, Korea cosmetic injections of the face. Plast Reconstr Surg 2012;129:995-1012. June-Gone Kim 4. Savino PJ, Burde RM, Mills RP. Visual loss following intra- Department of Ophthalmology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea nasal anesthetic injection. J Clin Neuroophthalmol E-mail: [email protected] 1990;10:140-4. 5. Webber B, Orlansky H, Lipton C, Stevens M. Complica- tions of an intra-arterial injection from an inferior alveolar Conflict of Interest nerve block. J Am Dent Assoc 2001;132:1702- 4. No potential conflict of interest relevant to this article was reported. Korean J Ophthalmol 2017;31(3):279-281 litus presented with blurred vision of the right eye for 10 https://doi.org/10.3341/kjo.2017.0002 days. Abdominal and chest computed tomography showed an emphysematous prostatic abscess with multiple pulmo- nary lesions. -

Acute Keratoconus-Like Corneal Hydrops Secondary to Ocular
perim Ex en l & ta a l ic O p in l h Journal of Clinical & Experimental t C h f a o l m l a o Ke et al., J Clin Exp Ophthalmol 2017, 8:6 n l o r g u y o Ophthalmology J DOI: 10.4172/2155-9570.1000694 ISSN: 2155-9570 Case Report Open Access Acute Keratoconus-Like Corneal Hydrops Secondary to Ocular Massage Following Trabeculectomy Hongmin Ke, Chengguo Zuo and Mingkai Lin* State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yat-sen University, 54 Xianlie Nan Road, Guangzhou, China *Corresponding author: Mingkai Lin, State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, 54 Xianlie Nan Road, Guangzhou, China, 510060, E- mail: [email protected] Received date: November 13, 2017; Accepted date: November 21, 2017; Published date: November 23, 2017 Copyright: © 2017 Ke H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Purpose: To report a case of acute keratoconus-like corneal hydrops in a patient with long-term ocular massage following trabeculectomy. Methods: Case report and review of medical literature. Results: A rare complication of acute keratoconus-like corneal hydrops occurred in a patient following the use of ocular massage to maintain satisfactory aqueous humor filtration after trabeculectomy. The patient had a history of high myopia but denied previous ocular trauma, allergic disease and a family history of keratoconus. Slit-lamp examination demonstrated keratoconus-like corneal hydrops with formation of epithelial microcystic, and intrastromal cleft. -

CAUSES, COMPLICATIONS &TREATMENT of A“RED EYE”
CAUSES, COMPLICATIONS & TREATMENT of a “RED EYE” 8 Most cases of “red eye” seen in general practice are likely to be conjunctivitis or a superficial corneal injury, however, red eye can also indicate a serious eye condition such as acute angle glaucoma, iritis, keratitis or scleritis. Features such as significant pain, photophobia, reduced visual acuity and a unilateral presentation are “red flags” that a sight-threatening condition may be present. In the absence of specialised eye examination equipment, such as a slit lamp, General Practitioners must rely on identifying these key features to know which patients require referral to an Ophthalmologist for further assessment. Is it conjunctivitis or is it something more Iritis is also known as anterior uveitis; posterior uveitis is serious? inflammation of the choroid (choroiditis). Complications include glaucoma, cataract and macular oedema. The most likely cause of a red eye in patients who present to 4. Scleritis is inflammation of the sclera. This is a very rare general practice is conjunctivitis. However, red eye can also be presentation, usually associated with autoimmune a feature of a more serious eye condition, in which a delay in disease, e.g. rheumatoid arthritis. treatment due to a missed diagnosis can result in permanent 5. Penetrating eye injury or embedded foreign body; red visual loss. In addition, the inappropriate use of antibacterial eye is not always a feature topical eye preparations contributes to antimicrobial 6. Acid or alkali burn to the eye resistance. The patient history will usually identify a penetrating eye injury Most general practice clinics will not have access to specialised or chemical burn to the eye, but further assessment may be equipment for eye examination, e.g. -

Hyphema. Part I. Pathophysiologic Considerations
University of Pennsylvania ScholarlyCommons Departmental Papers (Vet) School of Veterinary Medicine 11-1-1999 Hyphema. Part I. Pathophysiologic Considerations András M. Komáromy David T. Ramsey Dennis E. Brooks Cynthia C. Ramsey Maria E. Kallberg See next page for additional authors Follow this and additional works at: https://repository.upenn.edu/vet_papers Part of the Ophthalmology Commons, and the Veterinary Medicine Commons Recommended Citation Komáromy, A. M., Ramsey, D. T., Brooks, D. E., Ramsey, C. C., Kallberg, M. E., & Andrew, S. E. (1999). Hyphema. Part I. Pathophysiologic Considerations. Compendium on Continuing Education for the Practicing Veterinarian, 21 (11), 1064-1069. Retrieved from https://repository.upenn.edu/vet_papers/51 Dr. Komáromy was affiliated with the University of Pennsylvania from 2003-2012. Part II can be found at http://repository.upenn.edu/vet_papers/52/ This paper is posted at ScholarlyCommons. https://repository.upenn.edu/vet_papers/51 For more information, please contact [email protected]. Hyphema. Part I. Pathophysiologic Considerations Abstract Hemorrhage in the anterior chamber of the eye, or hyphema, results from a breakdown of the blood-ocular barrier (BOB) and is frequently associated with inflammation of the iris, ciliary body, or retina. Hyphema can also occur by retrograde blood flow into the anterior chamber via the aqueous humor drainage pathways without BOB breakdown. Hyphema attributable to blunt or perforating ocular trauma is more common than that resulting from endogenous causes. When trauma has been eliminated as a possible cause, it is prudent to assume that every animal with hyphema has a serious systemic disease until proven otherwise. Disciplines Medicine and Health Sciences | Ophthalmology | Veterinary Medicine Comments Dr. -
Diagnosis and Management of Common Eye Problems
Diagnosis and Management of Common Eye Problems Review of Ocular Anatomy Picture taken from Basic Ophthalmology for Medical Students and Primary Care Residents published by the American Academy of Ophthalmology Diagnosis and Management of Common Eye Problems Fernando Vega, MD Lacrimal system and eye musculature Eyelid anatomy Picture taken from Basic Ophthalmology for Medical Students and Primary Care Residents published by the American Academy of Ophthalmology n Red Eye Disorders: An Anatomical Approach n Lids n Orbit n Lacrimal System n Conjunctivitis n Cornea n Anterior Chamber Fernando Vega, MD 1 Diagnosis and Management of Common Eye Problems Red Eye Disorders: What is not in the scope of Red Eye Possible Causes of a Red Eye n Loss of Vision n Trauma n Vitreous Floaters n Chemicals n Vitreous detatchment n Infection n Retinal detachment n Allergy n Chronic Irritation n Systemic Infections Symptoms can help determine the Symptoms Continued diagnosis Symptom Cause Symptom Cause Itching allergy Deep, intense pain Corneal abrasions, scleritis Scratchiness/ burning lid, conjunctival, corneal Iritis, acute glaucoma, sinusitis disorders, including Photophobia Corneal abrasions, iritis, acute foreign body, trichiasis, glaucoma dry eye Halo Vision corneal edema (acute glaucoma, Localized lid tenderness Hordeolum, Chalazion contact lens overwear) Diagnostic steps to evaluate the patient with Diagnostic steps continued the red eye n Check visual acuity n Estimate depth of anterior chamber n Inspect pattern of redness n Look for irregularities in pupil size or n Detect presence or absence of conjunctival reaction discharge: purulent vs serous n Look for proptosis (protrusion of the globe), n Inspect cornea for opacities or irregularities lid malfunction or limitations of eye n Stain cornea with fluorescein movement Fernando Vega, MD 2 Diagnosis and Management of Common Eye Problems How to interpret findings n Decreased visual acuity suggests a serious ocular disease. -

Amaurosis Fugax (Transient Monocular Or Binocular Vision Loss)
Amaurosis fugax (transient monocular or binocular vision loss) Syndee Givre, MD, PhD Gregory P Van Stavern, MD The next version of UpToDate (15.3) will be released in October 2007. INTRODUCTION AND DEFINITIONS — Amaurosis fugax (from the Greek "amaurosis," meaning dark, and the Latin "fugax," meaning fleeting) refers to a transient loss of vision in one or both eyes. Varied use of common terminology may cause some confusion when reading the literature. Some suggest that "amaurosis fugax" implies a vascular cause for the visual loss, but the term continues to be used when describing visual loss from any origin and involving one or both eyes. The term "transient monocular blindness" is also often used but is not ideal, since most patients do not experience complete loss of vision with the episode. "Transient monocular visual loss" (TMVL) and "transient binocular visual loss" (TBVL) are preferred to describe abrupt and temporary loss of vision in one or both eyes, since they carry no connotation regarding etiology. Transient visual loss, either monocular or binocular, reflects a heterogeneous group of disorders, some relatively benign and others with grave neurologic or ophthalmologic implications. The task of the clinician is to use the history and examination to localize the problem to a region in the visual pathways, identify potential etiologies, and, when indicated, perform a focused battery of laboratory tests to confirm or exclude certain causes. Therapeutic interventions and prognostic implications are specific to the underlying cause. This topic discusses transient visual loss. Other ocular and cerebral ischemic syndromes are discussed separately. APPROACH TO TRANSIENT VISUAL LOSS — By definition, patients with transient visual loss almost always present after the episode has resolved; hence, the neurologic and ophthalmologic examination is usually normal. -

Eyelid and Orbital Emergencies Charles D
Eyelid and Orbital Emergencies Charles D. Rice M.D. Financial Disclosure Speaker, Charles Rice, M.D. has a financial interest/agreement or affiliation with Lansing Ophthalmology, where he is a shareholder and employed as a oculoplastic surgeon. Eyelid Emergencies/Urgencies • Chalazion with localized cellulitis • Preseptal Cellulitis • Contact Dermatitis • Canaliculitis • Dacryocystitis • Eyelid/Conjunctival Foreign Body Orbital Emergencies • Orbital Cellulitis • Orbital Inflammation • Thyroid Orbital Inflammation • Orbital Hemorrhage • Orbital and Eyelid Trauma Management • History • Exam Visual Acuity Pupillary Reaction Eyelid and Lacrimal Exam Globe position and Extraocular Motility Management • Diagnosis Differential Testing • Treatment Medications Surgery Referral Chalazion Chalazion with Localized Cellulitis • May be diffuse cellulitis • Usually painful • Consider dacryocystitis, canaliculitis, orbital cellulitis • Localized swelling and redness later Chalazion with Localized Cellulitis Treatment • Oral antibiotic Cephalosporin, Cipro, Bactrim • Topical antibiotic/steroid • Hot compresses • Incision and drainage later • 45 yo female • 1 month history of progressive redness and itching of eyelid area • Started on tobramycin and erythromycin topical • Benadryl • Lid scrubs • Problem continued to worsen Contact Dermatitis • Usually acute process • Redness, edema, flaking of skin • Unilateral or bilateral • Ocular exam usually normal • Exposure to chemicals or allergens • Pesticides, make-up, nail polish, plant materials • Consider bacterial