NUALS IP Law Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Fall-Out of a New Political Regime in India
ASIEN, (Juli 1998) 68, S. 5-20 The Fall-out of a New Political Regime in India Dietmar Rothermund Eine "rechte" Koalition geführt von der Bharatiya Janata Party (BJP) hat das "Congress System" abgelöst, das darin bestand, daß sich eine "Zentrumspartei" durch die Polarisation von rechter und linker Opposition an der Macht erhielt. Wahlallianzen der BJP haben regionalen Parteien zu Sitzen verholfen, die früher im Schatten der nationalen Parteien standen. So verschaffte sich die BJP Koali- tionspartner. Die Wähler haben der BJP kein überzeugendes Mandat erteilt, dennoch führte sie ihr Programm durch, zu dem die Atomtests gehörten, die dann in der Bevölkerung breite Zustimmung fanden. Die BJP fühlt sich so dazu ermutigt, eine sehr selbstbewußte Außenpolitik zu betreiben. Dazu gehört auch die Betonung der wirtschaftlichen Eigenständigkeit (Swadeshi). Der am 1.6.1998 vorgelegte Staatshaushalt zeigt kein Entgegenkommen gegenüber ausländischen Investoren. Den angekündigten amerikanischen Sanktionen wurde mit einer Rücklage begegnet und die Verteidigungsausgaben um 14 Prozent erhöht. The formation of a government led by the Bharatiya Janata Party (BJP) is certainly a new departure in Indian politics. All previous governments were formed either by the Indian National Congress or by politicians who had earlier belonged to that party. The BJP and its precursor the Bharatiya Jan Sangh had always been in the opposition with the exception of the brief interlude of the Janata government (1977- 1980) in which the present Prime Minister, Atal Bihari Vajpayee, held the post of Minister of External Affairs. The BJP has consistently stood for Hindu nationalism and has attacked the secularism of the Congress as a spurious ideology which should be replaced by genuine secularism. -

Leader of the House F
LEADER OF THE HOUSE F. No. RS. 17/5/2005-R & L © RAJYA SABHA SECRETARIAT, NEW DELHI http://parliamentofindia.nic.in http://rajyasabha.nic.in E-mail: [email protected] RAJYA SABHA SECRETARIAT PUBLISHED BY SECRETARY-GENERAL, RAJYA SABHA AND NEW DELHI PRINTED BY MANAGER, GOVERNMENT OF INDIA PRESS, MINTO ROAD, NEW DELHI-110002. PREFACE This booklet is part of the Rajya Sabha Practice and Procedure Series which seeks to describe, in brief, the importance, duties and functions of the Leader of the House. The booklet is intended to serve as a handy guide for ready reference. The information contained in it is synoptic and not exhaustive. New Delhi DR. YOGENDRA NARAIN February, 2005 Secretary-General THE LEADER OF THE HOUSE Leader of the House in Rajya Sabha Importance of the Office Rule 2(1) of the Rules of Procedure and Conduct of Business in the Council of States (Rajya Sabha) defines There are quite a few functionaries in Parliament who the Leader of Rajya Sabha as follows: render members’ participation in debates more real, effective and meaningful. One of them is the 'Leader of "Leader of the Council" means the Prime Minister, the House'. The Leader of the House is an important if he is a member of the Council, or a Minister who parliamentary functionary who exercises direct influence is a member of the Council and is nominated by the on the course of parliamentary business. Prime Minister to function as the Leader of the Council. Origin of Office in England In Rajya Sabha, the following members have served In England, one of the members of the Government, as the Leaders of the House since 1952: who is primarily responsible to the Prime Minister for the arrangement of the government business in the Name Period House of Commons, is known as the Leader of the House. -

WEDNESDAY, the 22ND JULY, 1998 (The Rajya Sabha Met in The
WEDNESDAY, THE 22ND JULY, 1998 (The Rajya Sabha met in the Parliament House at 11.00 a.m.) 1. STATEMENT BY MINISTER CORRECTING ANSWER TO QUESTION Shri L. K. Advani (Minister of Home Affairs) laid on the Table a Statement (in English and Hindi) correcting the reply given in Rajya Sabha on the 3rd June, 1998 to Unstarred Question 803 regarding Technicians in ITBP. 2. PAPERS LAID ON THE TABLE Shri P. R. Kumaramangalam (Minister of Power) laid on the Table a copy (in English and Hindi) of the Memorandum of Understanding between the Government of India (Ministry of Power) and National Hydro Electric Power Corporation Limited, for the year 1998-99. Shri Kashiram Rana (Minister of Textiles) laid on the Table- I. (1) A copy each (in English and Hindi) of the following papers, under sub-section (1) of section 619A of the Companies Act, 1956:- (i) Annual Report and Accounts of the Jute Corporation of India Limited, Calcutta, for the year 1996-97, together with the Auditors' Report on the Accounts and the comments of the Comptroller and Auditor General of India thereon. (ii) Review by Government on the working of the above Corporation. (2) Statement (in English and Hindi) giving reasons for the delay in laying the papers mentioned at (1) above. II. A copy each (in English and Hindi) of the following papers:- (i) Annual Report and Accounts of the Indian Jute Industries' Research Association, Calcutta, for the year 1996-97, together with the Auditors' Report on the Accounts. (ii) Review by Government on the working of the above Association. -

The Saffron Wave Meets the Silent Revolution: Why the Poor Vote for Hindu Nationalism in India
THE SAFFRON WAVE MEETS THE SILENT REVOLUTION: WHY THE POOR VOTE FOR HINDU NATIONALISM IN INDIA A Dissertation Presented to the Faculty of the Graduate School of Cornell University In Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy by Tariq Thachil August 2009 © 2009 Tariq Thachil THE SAFFRON WAVE MEETS THE SILENT REVOLUTION: WHY THE POOR VOTE FOR HINDU NATIONALISM IN INDIA Tariq Thachil, Ph. D. Cornell University 2009 How do religious parties with historically elite support bases win the mass support required to succeed in democratic politics? This dissertation examines why the world’s largest such party, the upper-caste, Hindu nationalist Bharatiya Janata Party (BJP) has experienced variable success in wooing poor Hindu populations across India. Briefly, my research demonstrates that neither conventional clientelist techniques used by elite parties, nor strategies of ideological polarization favored by religious parties, explain the BJP’s pattern of success with poor Hindus. Instead the party has relied on the efforts of its ‘social service’ organizational affiliates in the broader Hindu nationalist movement. The dissertation articulates and tests several hypotheses about the efficacy of this organizational approach in forging party-voter linkages at the national, state, district, and individual level, employing a multi-level research design including a range of statistical and qualitative techniques of analysis. In doing so, the dissertation utilizes national and author-conducted local survey data, extensive interviews, and close observation of Hindu nationalist recruitment techniques collected over thirteen months of fieldwork. BIOGRAPHICAL SKETCH Tariq Thachil was born in New Delhi, India. He received his bachelor’s degree in Economics from Stanford University in 2003. -

THURSDAY, the 30TH JULY, 1998 (The Rajya Sabha Met in The
THURSDAY, THE 30TH JULY, 1998 (The Rajya Sabha met in the Parliament House at 11.00 a.m.) 1. SHORT DURATION DISCUSSION Shri Kapil Sibal raised a discussion on the dismissal of the Government of Goa. Members took part in the discussion. The discussion was not concluded. (The House adjourned at 1.28 p.m. and re-assembled at 2.32 p.m.) (The House adjourned at 2.38 p.m. and re-assembled at 4.00 p.m.) (The House adjourned at 4.02 p.m. till 11.00 a.m. on Friday, the 31st, July, 1998). FRIDAY, THE 31ST JULY, 1998 (The Rajya Sabha met in the Parliament House at 11.00 a.m.) 1. STATEMENT BY MINISTER CORRECTING ANSWER TO QUESTION Shri Ramesh Bais (Minister of State in the Ministries of Steel & Mines) laid on the Table a Statement (in English and Hindi) correcting the reply given in the Rajya Sabha on the 13th July, 1998 to Unstarred Question 2852 regarding Officers and employees working in HSCC. Page-151 ;[31ST JULY, 1998] 2. PAPERS LAID ON THE TABLE Shri Sikander Bakht (Minister of Industry) on behalf of SHRI L. K. ADVANI laid on the Table a copy each of the following papers:- (i) First Report of the Jain Commission of Inquiry including its Annexures relating to assassination of Shri Rajiv Gandhi alongwith a Memorandum of Action Taken thereon. (ii) Explanatory Memorandum regarding not laying the Hindi version of the Final Report alongwith the English version and inability to prepare copies for circulation to all Members. Shri M. -

'State Visit' of Shri KR Narayanan, President of the Republic of India To
1 ‘State Visit’ of Shri KR Narayanan, President of the Republic of India to Peru and Brazil from 26 Apr to 10 May 1998 COMPOSITION OF DELEGATION (I) President and Family 1. The President 2. The First Lady 3. 2 x Daughter of the President 4. Granddaughter of the President (II) President’s Secretariat Delegation 1. Shri Gopalkrishna Gandhi Secretary to the President 2. Shri SK Sheriff Joint Secretary to the President 3. Maj Gen Bhopinder Singh Military Secretary to the President 4. Shri TP Seetharam Press Secretary to the President 5. Dr NK Khadiya Physician, President’s Estate Clinic No. of auxiliary staff : 15 (III) Parliamentary Delegation 1. Shri Ananth Kumar Minister of Civil Aviation 2. Shri Bangaru Laxman Member of Parliament (RS) 3. Shri Murli Deora Member of Parliament (LS) No. of supporting staff : 01 2 (IV) Ministry of External Affairs Delegation 1. Shri K Raghunath Foreign Secretary, MEA (for New York only) 2. Shri Lalit Mansingh Secretary (West), MEA 3. Shri Alok Prasad Joint Secretary (AMS), MEA (For New York only) 4. Shri SM Gavai Chief of Protocol, MEA 5. Smt Homai Saha Joint Secretary (LAC), MEA No. of supporting staff : 04 (V) Security Staff Total : 19 (VI) Media Delegation 1. Ms Rizwana Akhtar Correspondent, Doordarshan 2. Shri Sanjay Bhatnagar Correspondent, Univarta 3. Shri Ramesh Chand Cameraman, ANI 4. Dr John Cherian World Affairs Correspondent, Frontline 5. Shri Subhasish Choudhary Chief Recordist, Films Division 6. Ms Seema Goswami Editor, Weekend Magazine, Anand Bazar Patrika 7. Shri Mahesh Kamble Chief Cameraman, Films Division 8. Shri KL Katyal Programme Executive, All India Radio 3 9. -

MONDAY, the 3RD AUGUST, 1998 (The Rajya Sabha Met in The
MONDAY, THE 3RD AUGUST, 1998 (The Rajya Sabha met in the Parliament House at 11.00 a.m.) 1. OATH OR AFFIRMATION The following Members from the State of Haryana made and subscribed oath and took their seats in the House:- 1. Shri Devi Lal 2. Shri Swaraj Kaushal 2. PAPERS LAID ON THE TABLE Shri Sikander Bakht (Minister of Industry) on behalf of SHRI KASHIRAM RANA laid on the Table:- I. (1) A copy each (in English and Hindi) of the following papers under sub-section (1) of section 619A of the Companies Act, 1956:- (i) Twenty-ninth Annual Report and Accounts of the National Textile Corporation Limited, New Delhi, for the year 1996-97, together with the Auditors' Report on the Accounts and the comments of the Comptroller and Auditor General of India thereon. (ii) Review by Government on the Working of the above Corporation. (2) Statement (in English and Hindi) giving reasons for the delay in laying the papers mentioned at (I) above. Page-153 ;[3RD & 4TH AUG 1998] II. A copy each (in English and Hindi) of the following papers:- (a) Annual Report and Accounts of the Carpet Export Promotion Council, Noida (U.P.), for the year 1996-97, together with the Auditors' Report on the Accounts. (b) Review by Government on the working of the above Council. (c) Statement giving reasons for the delay in laying the papers mentioned at (a) above. 3. MESSAGE FROM THE LOK SABHA The Prasar Bharati (Broadcasting Corporation of India) Amendment Bill, 1998. Secretary-General reported to the House the following message received from the Lok Sabha, signed by the Secretary-General of the Lok Sabha: "In accordance with the provisions of rule 96 of the Rules or Procedure and Conduct of Business in Lok Sabha. -
![RAJYA SABHA] on the Table 244 12.00 NOON. [The Deputy](https://docslib.b-cdn.net/cover/9463/rajya-sabha-on-the-table-244-12-00-noon-the-deputy-2139463.webp)
RAJYA SABHA] on the Table 244 12.00 NOON. [The Deputy
243. Papers Laid (RAJYA SABHA] on the Table 244 PAPER LAID ON THE TABLE Notification of the Ministry of Communications THE MINISTER OF STATE IN THE MINISTRY OF POWER (SHRI P.V. RANGAYYA NAIDU): Madam, on behalf of Shri Sukh Ram, I lay on the Table, under subsection (5) of section 7 of the Indian Telegraph Act, 1885, a copy (in English and Hindi) of the Ministry of Communications (Department of Telecommunications) Notification G.S.R. No. 543 (E), dated the 28th June 1994, publishing the Indian Telegraph (Second Amendment) Rules, 1994. [Placed in Library. See No. LT. 6310/94] THE DEPUTY CHAIRMAN; Shri M.V. Chandrashekhar Murthy. (Interruptions). SHRI MD. SALIM (West Bengal): 12.00 NOON. Madam. I have a point of order. [The Deputy Chairman in the Chair] THE DEPUTY CHAIRMAN: What is your point? SHRI MD. SALIM: Madam, the hon STATEMENT BY MINISTER Minister, Shri M.V. Chandrashekhar Correcting answer to U.Q. No Murthy is going to lay on the Table of the 93 Answered on 26th July, House a copy of the Annual Report of the 1994 State Bank of India for the year 1993-94. On the 12th August, they had the Annual THE MINISTER OF STATE IN General Body Meeting in the Netaji THE MINISTRY OF FINANCE (SHRI Indoor Stadium, Calcutta, madam, now, M.V. CHANDRASHEKHAR PSU's are issuing shares to the public. MURTHY): Madam, I lay on the Table a Madam, many share holders are coming statement (in English and Hindi) and in SBI many old people are also there. correcting the reply given in the Rajya But, they have not made proper Sabha on the 26th July, 1994 to arrangements. -

MONDAY, the 7TH DECEMBER, 1998 (The Rajya Sabha Met in The
MONDAY, THE 7TH DECEMBER, 1998 (The Rajya Sabha met in the Parliament House at 11.00 a.m.) 1. PAPERS LAID ON THE TABLE Shri Sikander Bakht (Minister of Industry) laid on the Table- I. A copy (in English and Hindi) of the Ministry of Industry (Department of Industrial Deve- lopment) Notification No. G.S.R. 487(E), dated the 10th August, 1998, publishing the Gas Cylinder (Amendment) Rules, 1998, under section (8) of section 18 of the Explosives Act 1984. II. A copy (in English and Hindi) of the Ministry of Industry Notification S. O. 874(E), dated the 25th September, 1998 notifying the M/s. Kasat paper and Pulp Limited, Village Bebedohal, Taluka Maval,, District Pune in the estate of Maharasthra as a mill producing newsprint, under the Newsprint Control Order, 1962, under sub-section (3) of section 6 of the Essential Commodities Act, 1955. III. A copy each (in English and Hindi) of the following papers:- (i) (a) Twenty-eight Annual Report and Accounts of the Automotive Research Association on India, Pune, for the year 1997-98, together with the Auditors' Report on the Accounts. (b) Statement by Government accepting the above Report. (ii) (a) Thirty-seventh Annual Report and Accounts of the National Institute of Design, Ahmedabad, for the year 1997-98, together with the Auditors' Report on the Accounts. (b) Statement by Government accepting the above Report. (iii) (a) Annual Report and Accounts of the Institute for Design of Electrical Measuring Instruments, Mumbai, for the year 1997-98, together with the Auditors' Report on the Accounts. -

Padma Vibhushan * * the Padma Vibhushan Is the Second-Highest Civilian Award of the Republic of India , Proceeded by Bharat Ratna and Followed by Padma Bhushan
TRY -- TRUE -- TRUST NUMBER ONE SITE FOR COMPETITIVE EXAM SELF LEARNING AT ANY TIME ANY WHERE * * Padma Vibhushan * * The Padma Vibhushan is the second-highest civilian award of the Republic of India , proceeded by Bharat Ratna and followed by Padma Bhushan . Instituted on 2 January 1954, the award is given for "exceptional and distinguished service", without distinction of race, occupation & position. Year Recipient Field State / Country Satyendra Nath Bose Literature & Education West Bengal Nandalal Bose Arts West Bengal Zakir Husain Public Affairs Andhra Pradesh 1954 Balasaheb Gangadhar Kher Public Affairs Maharashtra V. K. Krishna Menon Public Affairs Kerala Jigme Dorji Wangchuck Public Affairs Bhutan Dhondo Keshav Karve Literature & Education Maharashtra 1955 J. R. D. Tata Trade & Industry Maharashtra Fazal Ali Public Affairs Bihar 1956 Jankibai Bajaj Social Work Madhya Pradesh Chandulal Madhavlal Trivedi Public Affairs Madhya Pradesh Ghanshyam Das Birla Trade & Industry Rajashtan 1957 Sri Prakasa Public Affairs Andhra Pradesh M. C. Setalvad Public Affairs Maharashtra John Mathai Literature & Education Kerala 1959 Gaganvihari Lallubhai Mehta Social Work Maharashtra Radhabinod Pal Public Affairs West Bengal 1960 Naryana Raghvan Pillai Public Affairs Tamil Nadu H. V. R. Iyengar Civil Service Tamil Nadu 1962 Padmaja Naidu Public Affairs Andhra Pradesh Vijaya Lakshmi Pandit Civil Service Uttar Pradesh A. Lakshmanaswami Mudaliar Medicine Tamil Nadu 1963 Hari Vinayak Pataskar Public Affairs Maharashtra Suniti Kumar Chatterji Literature -

JCC NUCLEAR ARMS RACE: INDIA BACKGROUND GUIDE & Letters from the Directors
&MUN IX JCC NUCLEAR ARMS RACE: INDIA BACKGROUND GUIDE & Letters From The Directors Dear Delegates, Welcome to &MUN IX and to the Nuclear Arms Race: India v. Pakistan. My name is Reeves Yanez and I will be the coordinating crisis director. I am a Junior at William & Mary majoring in Kinesiology with a concentration in Public Health. I have done MUN since middle school and since I have become increasingly involved, competing as part of W&M’s travel team, staffing our conferences, and serving as the USG for specialized agencies and Director General for our middle school conference, WMIDMUN. Outside of MUN, I love to spend time outside, leading students on backpacking trips through the student rec center. I am beyond excited to see what you all bring to the table as we discuss such an exciting topic with so many possibilities. I would encourage you to use history as a guide as you change the future of the subcontinent, but don’t be constrained by it.. The historical outcome was not perfect and I look forward to the alternate solutions you put forward. I especially value creativity and novel solutions, and plans that account for contingencies will be the most successful. With that being said there may be some sensitive subjects covered in this committee and we have high expectations of delegate conduct. We will not tolerate any racism, sexism, homophobia, transphobia, or any other form of discrimination. I look forward to your exciting plans as you strive to lead your nation to victory. This committee will also be a joint crisis committee, and so you will be actively working against another committee. -
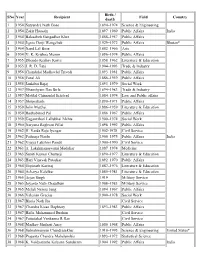
List of Padma Vibhushan Awardees
Birth / SNo Year Recipient Field Country death 1 1954 Satyendra Nath Bose 1894–1974 Science & Engineering 2 1954 Zakir Hussain 1897–1969 Public Affairs India 3 1954 Balasaheb Gangadhar Kher 1888–1957 Public Affairs 4 1954 Jigme Dorji Wangchuk 1929–1972 Public Affairs Bhutan* 5 1954 Nand Lal Bose 1882–1966 Arts 6 1954 V. K. Krishna Menon 1896–1974 Public Affairs 7 1955 Dhondo Keshav Karve 1858–1962 Literature & Education 8 1955 J. R. D. Tata 1904–1993 Trade & Industry 9 1956 Chandulal Madhavlal Trivedi 1893–1981 Public Affairs 10 1956 Fazal Ali 1886–1959 Public Affairs 11 1956 Jankibai Bajaj 1893–1979 Social Work 12 1957 Ghanshyam Das Birla 1894–1983 Trade & Industry 13 1957 Motilal Chimanlal Setalvad 1884–1974 Law and Public affairs 14 1957 Shriprakash 1890–1971 Public Affairs 15 1959 John Matthai 1886–1959 Literature & Education 16 1959 Radhabinod Pal 1886–1967 Public Affairs 17 1959 Gaganvihari Lallubhai Mehta 1900–1974 Social Work 18 1960 Naryana Raghvan Pillai 1898–1992 Public Affairs 19 1962 H. Varda Raja Iyengar 1902-1978 Civil Service 20 1962 Padmaja Naidu 1900–1975 Public Affairs India 21 1962 Vijaya Lakshmi Pandit 1900–1990 Civil Service 22 1963 A. Lakshmanaswami Mudaliar 1887–1974 Medicine 23 1963 Suniti Kumar Chatterji 1890–1977 Literature & Education 24 1963 Hari Vinayak Pataskar 1892–1970 Public Affairs 25 1964 Gopinath Kaviraj 1887–1976 Literature & Education 26 1964 Acharya Kalelkar 1885–1981 Literature & Education 27 1965 Arjan Singh 1919 Military Service 28 1965 Joyanto Nath Chaudhuri 1908–1983 Military Service 29 1965 Mehdi Nawaz Jung 1894–1967 Public Affairs 30 1966 Valerian Gracias 1900–1978 Social Work 31 1967 Bhola Nath Jha Civil Service 32 1967 Chandra Kisan Daphtary 1893–1983 Public Affairs 33 1967 Hafix Mohammed Ibrahim Civil Service 34 1967 Pattadakal Venkanna R Rao Civil Service 35 1968 Madhav Shrihari Aney 1880–1968 Public Affairs 36 1968 Subrahmanyan Chandrasekhar 1910–1995 Science & Engineering United States* 37 1968 Prasanta Chandra Mahalanobis 1893–1972 Statistical Science 38 1968 K.