Optimizing the Use of Topical Agents in Psoriasis Linda F
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

This Fact Sheet Provides Information to Patients with Eczema and Their Carers. About Topical Corticosteroids How to Apply Topic
This fact sheet provides information to patients with eczema and their carers. About topical corticosteroids You or your child’s doctor has prescribed a topical corticosteroid for the treatment of eczema. For treating eczema, corticosteroids are usually prepared in a cream or ointment and are applied topically (directly onto the skin). Topical corticosteroids work by reducing inflammation and helping to control an over-reactive response of the immune system at the site of eczema. They also tighten blood vessels, making less blood flow to the surface of the skin. Together, these effects help to manage the symptoms of eczema. There is a range of steroids that can be used to treat eczema, each with different strengths (potencies). On the next page, the potencies of some common steroids are shown, as well as the concentration that they are usually used in cream or ointment preparations. Using a moisturiser along with a steroid cream does not reduce the effect of the steroid. There are many misconceptions about the side effects of topical corticosteroids. However these treatments are very safe and patients are encouraged to follow the treatment regimen as advised by their doctor. How to apply topical corticosteroids How often should I apply? How much should I apply? Apply 1–2 times each day to the affected area Enough cream should be used so that the of skin according to your doctor’s instructions. entire affected area is covered. The cream can then be rubbed or massaged into the Once the steroid cream has been applied, inflamed skin. moisturisers can be used straight away if needed. -

Etats Rapides
List of European Pharmacopoeia Reference Standards Effective from 2015/12/24 Order Reference Standard Batch n° Quantity Sale Information Monograph Leaflet Storage Price Code per vial Unit Y0001756 Exemestane for system suitability 1 10 mg 1 2766 Yes +5°C ± 3°C 79 ! Y0001561 Abacavir sulfate 1 20 mg 1 2589 Yes +5°C ± 3°C 79 ! Y0001552 Abacavir for peak identification 1 10 mg 1 2589 Yes +5°C ± 3°C 79 ! Y0001551 Abacavir for system suitability 1 10 mg 1 2589 Yes +5°C ± 3°C 79 ! Y0000055 Acamprosate calcium - reference spectrum 1 n/a 1 1585 79 ! Y0000116 Acamprosate impurity A 1 50 mg 1 3-aminopropane-1-sulphonic acid 1585 Yes +5°C ± 3°C 79 ! Y0000500 Acarbose 3 100 mg 1 See leaflet ; Batch 2 is valid until 31 August 2015 2089 Yes +5°C ± 3°C 79 ! Y0000354 Acarbose for identification 1 10 mg 1 2089 Yes +5°C ± 3°C 79 ! Y0000427 Acarbose for peak identification 3 20 mg 1 Batch 2 is valid until 31 January 2015 2089 Yes +5°C ± 3°C 79 ! A0040000 Acebutolol hydrochloride 1 50 mg 1 0871 Yes +5°C ± 3°C 79 ! Y0000359 Acebutolol impurity B 2 10 mg 1 -[3-acetyl-4-[(2RS)-2-hydroxy-3-[(1-methylethyl)amino] propoxy]phenyl] 0871 Yes +5°C ± 3°C 79 ! acetamide (diacetolol) Y0000127 Acebutolol impurity C 1 20 mg 1 N-(3-acetyl-4-hydroxyphenyl)butanamide 0871 Yes +5°C ± 3°C 79 ! Y0000128 Acebutolol impurity I 2 0.004 mg 1 N-[3-acetyl-4-[(2RS)-3-(ethylamino)-2-hydroxypropoxy]phenyl] 0871 Yes +5°C ± 3°C 79 ! butanamide Y0000056 Aceclofenac - reference spectrum 1 n/a 1 1281 79 ! Y0000085 Aceclofenac impurity F 2 15 mg 1 benzyl[[[2-[(2,6-dichlorophenyl)amino]phenyl]acetyl]oxy]acetate -

120.Clobetasol Propionate and [email protected]
IAJPS 2017, 4 (10), 3779-3786 Harikishor Barange et al ISSN 2349-7750 CODEN (USA): IAJPBB ISSN: 2349-7750 INDO AMERICAN JOURNAL OF PHARMACEUTICALSCIENCES Available online at: http://www.iajps.com Research Article DEVELOPMENT OF ANALYTICAL METHOD AND VALIDATION FOR SIMULTANEOUS ESTIMATION OF CLOBETASOL PROPIONATE AND KETOCONAZOLE IN PHARMACEUTICAL CREAM FORMULATION BY RP-HPLC METHOD Harikishor Barange*, Suhail Asghar and Pooja Gour Unijules Life Sciences Ltd., B - 35, 36 MIDC Area Kalmeshwar Nagpur – 441501, Maharashtra, India. E-mail of Corresponding author: [email protected]. Abstract: A simple, accurate rapid and precise has been developed and validated for simultaneous estimation of Clobetasol Propionate and Ketoconazole in Pharmaceutical Cream Formulation by RP-HPLC Method. The successful estimation was carried out of the drug product is developed on the ODS Qualisil Column C18 (250 mm x 4.6 mm, 5μm or equivalent) at ambient temperature using Methanol: Acetonitrile: Phosphate buffer (50:20:30 v/v/v) as mobile phase composition. The flow rate was adjusted to 1.5mL/minute and the absorption maxima were observed on UV detector at 254 nm. Retention Time for Clobetasole Propionate 11.1±0.1min and Ketoconazole 12.2±0.1min. The linearity was obtained in the concentration range of 6-14µg/mL and 120-280µg/mL for Clobetasole Propionate and Ketoconazole respectively. Mean percentage recoveries were 99.16% for Clobetasole Propionate and 99.17% for Ketoconazole. The LOD of Clobetasole Propionate and Ketoconazole were found to be 0.7μg/mL and 2.0μg/mL whereas the LOQ was 3.0μg/mL and 10.0μg/mL respectively. Percentage relative standard deviation of percent assay values for replicate sample preparation was 0.28% for Clobetasole Propionate and 0.47% for Ketoconazole. -

St John's Institute of Dermatology
St John’s Institute of Dermatology Topical steroids This leaflet explains more about topical steroids and how they are used to treat a variety of skin conditions. If you have any questions or concerns, please speak to a doctor or nurse caring for you. What are topical corticosteroids and how do they work? Topical corticosteroids are steroids that are applied onto the skin and are used to treat a variety of skin conditions. The type of steroid found in these medicines is similar to those produced naturally in the body and they work by reducing inflammation within the skin, making it less red and itchy. What are the different strengths of topical corticosteroids? Topical steroids come in a number of different strengths. It is therefore very important that you follow the advice of your doctor or specialist nurse and apply the correct strength of steroid to a given area of the body. The strengths of the most commonly prescribed topical steroids in the UK are listed in the table below. Table 1 - strengths of commonly prescribed topical steroids Strength Chemical name Common trade names Mild Hydrocortisone 0.5%, 1.0%, 2.5% Hydrocortisone Dioderm®, Efcortelan®, Mildison® Moderate Betamethasone valerate 0.025% Betnovate-RD® Clobetasone butyrate 0.05% Eumovate®, Clobavate® Fluocinolone acetonide 0.001% Synalar 1 in 4 dilution® Fluocortolone 0.25% Ultralanum Plain® Fludroxycortide 0.0125% Haelan® Tape Strong Betamethasone valerate 0.1% Betnovate® Diflucortolone valerate 0.1% Nerisone® Fluocinolone acetonide 0.025% Synalar® Fluticasone propionate 0.05% Cutivate® Hydrocortisone butyrate 0.1% Locoid® Mometasone furoate 0.1% Elocon® Very strong Clobetasol propionate 0.1% Dermovate®, Clarelux® Diflucortolone valerate 0.3% Nerisone Forte® 1 of 5 In adults, stronger steroids are generally used on the body and mild or moderate steroids are used on the face and skin folds (armpits, breast folds, groin and genitals). -

Clobetasol Propionate Lotion in the Treatment of Moderate to Severe Plaque-Type Psoriasis
THERAPEUTICS FOR THE CLINICIAN Clobetasol Propionate Lotion in the Treatment of Moderate to Severe Plaque-Type Psoriasis Jacques Decroix, MD; Henrik Pres, MD; Nicolaï Tsankov, MD; Michel Poncet, PhD; Stéphanie Arsonnaud Owing to its anti-inflammatory, antipruritic, soriasis is a lifelong condition, with onset vasoconstrictive, and immune-modulating prop- occurring at any time throughout life. It erties, clobetasol propionate is used to treat P affects men and women equally, and almost all psoriasis. This study was conducted to evaluate races in varying rates of frequency are affected. Pso- the efficacy, safety, and cosmetic acceptability riasis usually first appears between the ages of 15 of clobetasol propionate lotion compared with and 30 years and may occur anywhere on the body. its vehicle and with clobetasol propionate Psoriasis is an inherited condition; however, cream in the treatment of moderate to severe both genetic and environmental factors play an plaque-type psoriasis. important role in its onset and course. The condi- A total of 222 patients were treated. After tion has a considerable impact on quality of life, 4 weeks of treatment, clobetasol propionate with patients complaining about the messiness of lotion was more efficient than vehicle lotion and the topical agents used to treat the condition and of equivalent efficacy as clobetasol propionate the profound psychological impact of the treat- cream. Cosmetic acceptability was significantly ments and the condition.1-5 better with clobetasol propionate lotion than with Clobetasol propionate is known for its anti- clobetasol propionate cream. Clobetasol propi- inflammatory, antipruritic, vasoconstrictive, and onate lotion was efficient, safe, and well toler- immune-modulating properties and is currently ated and offers a significantly higher cosmetic used in the treatment of certain hyperproliferative advantage in the treatment of moderate to or inflammatory dermatoses, including psoriasis severe plaque-type psoriasis compared with and atopic dermatitis. -

Connecticut Medicaid
ACNE AGENTS, TOPICAL ‡ ANGIOTENSIN MODULATOR COMBINATIONS ANTICONVULSANTS, CONT. CONNECTICUT MEDICAID (STEP THERAPY CATEGORY) AMLODIPINE / BENAZEPRIL (ORAL) LAMOTRIGINE CHEW DISPERS TAB (not ODT) (ORAL) (DX CODE REQUIRED - DIFFERIN, EPIDUO and RETIN-A) AMLODIPINE / OLMESARTAN (ORAL) LAMOTRIGINE TABLET (IR) (not ER) (ORAL) Preferred Drug List (PDL) ACNE MEDICATION LOTION (BENZOYL PEROXIDE) (TOPICAL)AMLODIPINE / VALSARTAN (ORAL) LEVETIRACETAM SOLUTION, IR TABLET (not ER) (ORAL) • The Connecticut Medicaid Preferred Drug List (PDL) is a BENZOYL PEROXIDE CREAM, WASH (not FOAM) (TOPICAL) OXCARBAZEPINE TABLET (ORAL) listing of prescription products selected by the BENZOYL PEROXIDE 5% and 10% GEL (OTC) (TOPICAL) ANTHELMINTICS PHENOBARBITAL ELIXIR, TABLET (ORAL) Pharmaceutical and Therapeutics Committee as efficacious, BENZOYL PEROXIDE 6% CLEANSER (OTC) (TOPICAL) ALBENDAZOLE TABLET (ORAL) PHENYTOIN CHEW TABLET, SUSPENSION (ORAL) safe and cost effective choices when prescribing for HUSKY CLINDAMYCIN PH 1% PLEGET (TOPICAL) BILTRICIDE TABLET (ORAL) PHENYTOIN SOD EXT CAPSULE (ORAL) A, HUSKY C, HUSKY D, Tuberculosis (TB) and Family CLINDAMYCIN PH 1% SOLUTION (not GEL or LOTION) (TOPICAL)IVERMECTIN TABLET (ORAL) PRIMIDONE (ORAL) Planning (FAMPL) clients. CLINDAMYCIN / BENZOYL PEROXIDE 1.2%-5% (DUAC) (TOPICAL) SABRIL 500 MG POWDER PACK (ORAL) • Preferred or Non-preferred status only applies to DIFFERIN 0.1% CREAM (TOPICAL) (not OTC GEL) (DX CODE REQ.) ANTI-ALLERGENS, ORAL SABRIL TABLET (ORAL) those medications that fall within the drug classes DIFFERIN -

An Open Label Study of Clobetasol Propionate 0.05% and Betamethasone Valerate 0.12% Foams in the Treatment of Mild to Moderate Acne Keloidalis
HIGHLIGHTING SKIN OF COLOR An Open Label Study of Clobetasol Propionate 0.05% and Betamethasone Valerate 0.12% Foams in the Treatment of Mild to Moderate Acne Keloidalis Valerie D. Callender, MD; Cherie M. Young, MD; Christina L. Haverstock, MD; Christie L. Carroll, MD; Steven R. Feldman, MD, PhD Acne keloidalis (AK) is a disease affecting pri- 1942.2,3 It is predominantly a condition of African marily African American men. Topical steroids American men4; however, it also occurs in African are a widely accepted treatment of AK; however, American women5 and other ethnic groups. The no studies have been published investigating true incidence of AK is varied, and studies suggest their effectiveness. The purpose of this open- a range of 0.45% to 13.7% in blacks.6-8 Studies per- label study was to assess the efficacy and toler- formed by Halder et al9 and Kenny10 did not find ability of clobetasol propionate 0.05% and AK to be in the 12 most common diagnoses in betamethasone valerate 0.12% foams in the African Americans. treatment of AK in 20 African American patients. AK begins as papules and pustules on the occip- These patients were treated for 8 to 12 weeks ital scalp and posterior neck that may develop into using a pulsed-dose regimen. We found topical nodules or coalesce into plaques. In some cases, clobetasol propionate foam to be effective in other areas of the scalp may be involved, including improving AK, and our patients found the foam the vertex. Initially, hair shafts can be seen exiting vehicle to be cosmetically acceptable. -

Step Therapy
UnitedHealthcare Pharmacy Clinical Pharmacy Programs Program Number 2021 P 3033-10 Program Step Therapy – Topical Steroids Medication Topical Steroids: Cordran (flurandrenolide) cream 0.05%, Cordran (flurandrenolide) lotion 0.05%, Cordran (flurandrenolide) ointment 0.05%, Cloderm (clocortolone pivalate) cream 0.1%, Halog (halcinonide) cream 0.1%, Halog (halcinonide) ointment 0.1%, Desonate (desonide gel) gel 0.05% Cultivate lotion (fluticasone propionate 0.05% lotion), Ultravate (halobetasol propionate) lotion 0.05%, Bryhali (halobetasol propionate) lotion 0.01% P&T Approval Date 8/2014, 7/2015, 10/2015, 3/2016, 1/2017, 2/2018, 2/2019, 3/2020, 6/2021 Effective Date 9/1/2021; Oxford only: 9/1/2021 1. Background: Topical steroids are commonly prescribed for the treatment of rash, eczema, and dermatitis. Topical steroids have anti-inflammatory properties, and are classified into different potency classes based on their vasoconstriction abilities. A vasoconstriction bioassay provides potency measurements that correlate with clinical potency. There are numerous topical steroid products. Step Therapy programs are utilized to encourage the use of lower cost alternatives for certain therapeutic classes. This program requires a member to try one or two lower cost alternative topical steroids before providing coverage for higher cost topical steroids. Generic equivalent medications for brands listed as a step 2 agent will also be targeted when available. Class 1: Super Potent Class 5: Lower Mid-Strength Class 2: Potent Class 6: Mild Class 3: Upper Mid- Strength Class 7: Least Potent Class 4:Mid-Strength © 2021 UnitedHealthcare Services Inc. 1 2. Coverage Criteria a: A. Topical steroids will be approved based on the following criterion: 1. -

Clobetasol Propionate Topical Solution USP, 0.05%
CLOBETASOL PROPIONATE- clobetasol propionate solution Taro Pharmaceuticals U.S.A., Inc. ---------- Clobetasol Propionate Topical Solution USP, 0.05% Rx only FOR TOPICAL DERMATOLOGIC USE ONLY – NOT FOR OPHTHALMIC, ORAL OR INTRAVAGINAL USE. DESCRIPTION Clobetasol Propionate Topical Solution USP, 0.05% contains the active compound clobetasol propionate, a synthetic corticosteroid, for topical dermatologic use. Clobetasol, an analog of prednisolone, has a high degree of glucocorticoid activity and a slight degree of mineralocorticoid activity. Chemically, clobetasol propionate is (11β,16β)-21-chloro-9-fluoro-11-hydroxy-16-methyl-17-(1- oxopropoxy)pregna-1,4-diene-3,20-dione and it has the following structural formula: Clobetasol propionate has the molecular formula C25H32ClFO5 and a molecular weight of 467. It is a white to cream-colored crystalline powder insoluble in water. Clobetasol propionate topical solution contains clobetasol propionate 0.5 mg/g in a base composed of carbomer 934P, isopropyl alcohol (39.3%), purified water, and sodium hydroxide. CLINICAL PHARMACOLOGY The corticosteroids are a class of compounds comprising steroid hormones secreted by the adrenal cortex and their synthetic analogs. In pharmacologic doses, corticosteroids are used primarily for their anti-inflammatory and/or immunosuppressive effects. Topical corticosteroids such as clobetasol propionate are effective in the treatment of corticosteroid-responsive dermatoses primarily because of their anti-inflammatory, antipruritic, and vasoconstrictive actions. However, while the physiologic, pharmacologic, and clinical effects of the corticosteroids are well known, the exact mechanisms of their actions in each disease are uncertain. Clobetasol propionate, a corticosteroid, has been shown to have topical (dermatologic) and systemic pharmacologic and metabolic effects characteristic of this class of drugs. -

Alexander Weizel.Pdf
Target Analysis of a Large Number of Steroid Hormones Corticosteroids and Progestogens in Wastewater Treatment Plants and Receiving Surface Waters Alexander Weizel, M. Schlüsener, G. Dierkes, T. A. Ternes Federal Institute of Hydrology, Koblenz Germany Water JPI Conference 06/06/18 - 07/06/18 Helsinki Overview • Aims of the research • Development of an analytical method (down to 0.02 ng/L) • Determination of steroidal pollutants in WWTP effluents and surface waters Weizel A. et al., Environ. Sci. Technol. 2018, 52, 5296−5307. Substance Defined Daily Dose* Class Carbamazepine 1000 mg Anti-epileptic Spironolactone 75 mg Mineralocorticoid Dienogest 2 mg Progestogen Fluticasone propionate 0.2 mg Glucocorticoid *whocc.no. ATC/DDDIndex. https://www.whocc.no. Water JPI Conference, 07/06/18, Helsinki Alexander Weizel 2 Analysis of Steroid Hormones • Target Compound Selection based on • Prescription numbers in Germany, metabolism ➢37 Glucocorticoids ➢18 Progestogens ➢5 Mineralocorticoids Sample Preparation ESI-LC-MS/MS 1. Solid Phase 2. Clean-Up 3. Chromatographic 4. Quantification via Extraction Separation by RP-LC ESI-Quadrupole-MS/MS Water JPI Conference, 07/06/18, Helsinki Alexander Weizel 3 Target Analytes - Mineralocorticoids Mineralocorticoids Regulation of K+/Na+ balance• Synthesis in the adernal cortex (Corticoids) • Medical Indication • Medical indication • Diuretics • Regulation of the K/Na balance • Addison‘s disease • Diuretic • High blood pressure • Addison‘s disease Aldosterone (natural) Consumption in GER (2014): 9150 kg Spironolactone -
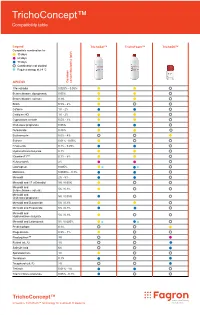
Trichoconcept™ Compatibility Table
TrichoConcept™ Compatibility table Legend TrichoSol™ TrichoFoam™ TrichoOil™ Compatible combination for 30 days 60 days 90 days Combination not studied Requires storage at 2-8 °C API/DCI Common concentration(s) (w/v) 17α-estradiol 0.025% - 0.05% Betamethasone dipropionate 0.05% Betamethasone valerate 0.1% Biotin 0.5% - 2% Caffeine 1% - 2% Cetirizine HCl 1% - 2% Cyproterone acetate 0.5% - 1% Clobetasol propionate 0.05% Dutasteride 0.25% Erythromycin 0.5% - 4% Estrone 0.01% - 0.05% Finasteride 0.1% - 0.25% Hydrocortisone butyrate 0.1% IGrantineF1™ 0.1% - 5% Ketoconazole 2% Latanoprost 0.005% Melatonin 0.0033% - 0.1% Minoxidil 2% - 5% Minoxidil and 17-α Estradiol 5% / 0.05% Minoxidil and 5% / 0.1% Betamethasone valerate Minoxidil and 5% / 0.05% Clobetasol propionate Minoxidil and Dutasteride 5% / 0.1% Minoxidil and Finasteride 5% / 0.1% Minoxidil and 5% / 0.1% Hydrocortisone butyrate Minoxidil and Latanoprost 5% / 0.005% Prednicarbate 0.1% Progesterone 0.5% - 1% Prostaquinon™ 3% Retinol (vit. A) 1% Salicylic Acid 6% Spironolactone 1% Tacrolimus 0.1% Tocopherol (vit. E) 2% Tretinoin 0.01% - 1% Triamcinolone acetonide 0.05% - 0.1% TrichoConcept™ Innovative TrichoTech™ technology for treatment of alopecia Fagron Hellas 12 km N.R. Trikala - Larisa T +30 24310 83633-5 P.C. 42100, P.O. Box 32 F +30 24310 83615 Trikala, Greece www.fagron.gr TRICHOCT02 herein. contained information or formulations the for case any in liable or responsible held be cannot and accept not does Fagron judgement. and table cannot be construed as (medical) advice, recommendation or opinion. Medical professionals, doctors and compounding pharmacists using this information are advised to do so solely if appropriate in their own professional opinion opinion professional own their in appropriate if solely so do to advised are information this using pharmacists compounding and doctors professionals, Medical opinion. -

Halobetasol (ULTRAVATE) Lotion Cream Ointment Monograph
Halobetasol Propionate (ULTRAVATE) Lotion 0.05%, and Halobetasol Propionate Cream and Ointment National Drug Monograph October 2016 VA Pharmacy Benefits Management Services, Medical Advisory Panel, and VISN Pharmacist Executives The purpose of VA PBM Services drug monographs is to provide a focused drug review for making formulary decisions. Updates will be made when new clinical data warrant additional formulary discussion. Documents will be placed in the Archive section when the information is deemed to be no longer current. FDA Approval Information Description/Mechanism Halobetasol propionate is a corticosteroid that plays a role in cellular signaling, of Action immune function, inflammation, and protein regulation; however, the precise mechanism of action in plaque psoriasis is unknown. Halobetasol propionate has a trihalogenated chemical structure that is similar to that of clobetasol propionate. Halobetasol propionate lotion is a super-high potency corticosteroid product. Indication(s) Under Lotion: Topical treatment of plaque psoriasis in patients eighteen (18) years of age Review in This and older Document Cream and Ointment: Relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses. Dosage Form(s) Under The primary formulation under review is halobetasol propionate lotion 0.05%. Review Each gram of lotion contains 0.5 mg of halobetasol propionate. Secondary formulations under review: Cream 0.05% and Ointment 0.05% REMS REMS No REMS Postmarketing Requirements See Other Considerations for additional REMS information Pregnancy Rating Lotion: No data in pregnant women. Cream and Ointment: Category C Executive Summary Efficacy Halobetasol propionate lotion had a moderate to large effect size in achieving overall treatment success (NNTs of 2.6 and 2.7) in two major efficacy-safety trials.