Membrane Dynamics: MIEF1 Mingles with Mitochondria
Total Page:16
File Type:pdf, Size:1020Kb
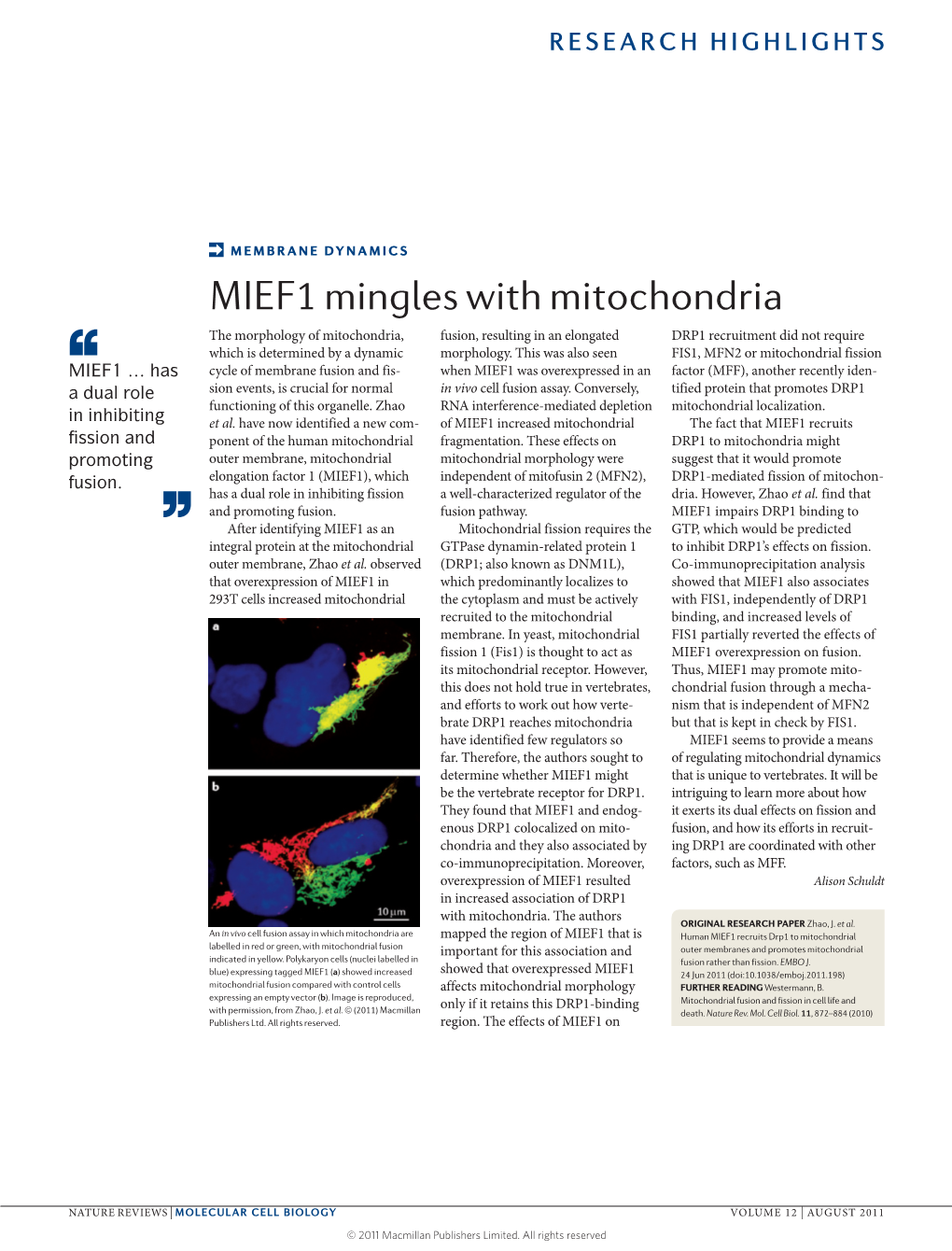
Load more
Recommended publications
-

Mitochondrial Rab Gaps Govern Autophagosome Biogenesis During Mitophagy Koji Yamano1, Adam I Fogel1, Chunxin Wang1, Alexander M Van Der Bliek2, Richard J Youle1*
RESEARCH ARTICLE elife.elifesciences.org Mitochondrial Rab GAPs govern autophagosome biogenesis during mitophagy Koji Yamano1, Adam I Fogel1, Chunxin Wang1, Alexander M van der Bliek2, Richard J Youle1* 1Biochemistry Section, Surgical Neurology Branch, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, United States; 2Department of Biological Chemistry, David Geffen School of Medicine at University of California, Los Angeles, Los Angeles, United States Abstract Damaged mitochondria can be selectively eliminated by mitophagy. Although two gene products mutated in Parkinson’s disease, PINK1, and Parkin have been found to play a central role in triggering mitophagy in mammals, how the pre-autophagosomal isolation membrane selectively and accurately engulfs damaged mitochondria remains unclear. In this study, we demonstrate that TBC1D15, a mitochondrial Rab GTPase-activating protein (Rab-GAP), governs autophagosome biogenesis and morphology downstream of Parkin activation. To constrain autophagosome morphogenesis to that of the cargo, TBC1D15 inhibits Rab7 activity and associates with both the mitochondria through binding Fis1 and the isolation membrane through the interactions with LC3/ GABARAP family members. Another TBC family member TBC1D17, also participates in mitophagy and forms homodimers and heterodimers with TBC1D15. These results demonstrate that TBC1D15 and TBC1D17 mediate proper autophagic encapsulation of mitochondria by regulating Rab7 activity at the interface between mitochondria and isolation membranes. DOI: 10.7554/eLife.01612.001 *For correspondence: [email protected] Introduction Competing interests: See page 21 Autophagosomes enclose seemingly random portions of the cytoplasm to supply nutrients during starvation or they can specifically engulf cellular debris to maintain quality control (Mizushima et al., Funding: See page 21 2011). -

Differential Redox-Regulation and Mitochondrial Dynamics in Normal and Leukemic Hematopoietic Stem Cells a Potential Window
Critical Reviews in Oncology / Hematology 144 (2019) 102814 Contents lists available at ScienceDirect Critical Reviews in Oncology / Hematology journal homepage: www.elsevier.com/locate/critrevonc Differential redox-regulation and mitochondrial dynamics in normal and leukemic hematopoietic stem cells: A potential window for leukemia T therapy ⁎ Katharina Mattes, Edo Vellenga, Hein Schepers Department of Hematology, Cancer Research Center Groningen, University Medical Center Groningen, University of Groningen, Groningen, the Netherlands ARTICLE INFO ABSTRACT Keywords: The prognosis for many patients with acute myeloid leukemia (AML) is poor, mainly due to disease relapse HSC driven by leukemia stem cells (LSCs). Recent studies have highlighted the unique metabolic properties of LSCs, LSC which might represent opportunities for LSC-selective targeting. LSCs characteristically have low levels of re- Mitochondria active oxygen species (ROS), which apparently result from a combination of low mitochondrial activity and high ROS activity of ROS-removing pathways such as autophagy. Due to this low activity, LSCs are highly dependent on BCL-2 mitochondrial regulatory mechanisms. These include the anti-apoptotic protein BCL-2, which also has crucial Autophagy Venetoclax roles in regulating the mitochondrial membrane potential, and proteins involved in mitophagy. Here we review the different pathways that impact mitochondrial activity and redox-regulation, and highlight their relevance for the functionality of both HSCs and LSCs. Additionally, novel AML therapy strategies that are based on interference with those pathways, including the promising BCL-2 inhibitor Venetoclax, are summar- ized. 1. Introduction and scope of this review regulating ROS production and mitochondrial health. HSCs rely on glycolysis as their main energy source, which results in less oxidative Current treatment strategies for acute myeloid leukemia (AML) re- burden compared to mitochondrial oxidative phosphorylation (OX- sult in an initial reduction of leukemic blasts in the majority of patients. -

Appoptosin Interacts with Mitochondrial Outer-Membrane Fusion Proteins and Regulates Mitochondrial Morphology
© 2016. Published by The Company of Biologists Ltd | Journal of Cell Science (2016) 129, 994-1002 doi:10.1242/jcs.176792 RESEARCH ARTICLE Appoptosin interacts with mitochondrial outer-membrane fusion proteins and regulates mitochondrial morphology Cuilin Zhang1, Zhun Shi1, Lingzhi Zhang1, Zehua Zhou1, Xiaoyuan Zheng1, Guiying Liu1, Guojun Bu1, Paul E. Fraser2, Huaxi Xu1,3 and Yun-wu Zhang1,* ABSTRACT mitochondrial mobility, mitophagy, cell mitosis and apoptosis Mitochondrial morphology is regulated by fusion and fission (Youle and van der Bliek, 2012). – machinery. Impaired mitochondria dynamics cause various Mitochondrial fusion comprises two events outer-membrane diseases, including Alzheimer’s disease. Appoptosin (encoded by fusion and inner-membrane fusion. In mammalian cells, two SLC25A38) is a mitochondrial carrier protein that is located in the GTPases MFN1 and MFN2, which anchor on the outer mitochondrial inner membrane. Appoptosin overexpression causes membrane of mitochondria, are responsible for the outer- overproduction of reactive oxygen species (ROS) and caspase- membrane fusion (Chen et al., 2003; Koshiba et al., 2004). dependent apoptosis, whereas appoptosin downregulation abolishes However, compared to MFN2, MFN1 is relatively specific in β-amyloid-induced mitochondrial fragmentation and neuronal death regulating mitochondrial fusion (Shen et al., 2007). MFN1- during Alzheimer’s disease. Herein, we found that overexpression harboring mitochondria have a higher tethering efficiency than of appoptosin resulted in mitochondrial fragmentation in a manner those with MFN2, and purified MFN1 also possesses higher independent of its carrier function, ROS production or caspase GTPase activity than MFN2 (Ishihara et al., 2004). In contrast, activation. Although appoptosin did not affect levels of mitochondrial MFN2 is more tissue specific in its expression pattern than MFN1 outer-membrane fusion (MFN1 and MFN2), inner-membrane fusion (Liesa et al., 2009). -

Regulation of Mitochondrial Dynamics and Cell Fate Rimpy Dhingra, Phd; Lorrie A
Advance Publication by-J-STAGE Circulation Journal REVIEW Official Journal of the Japanese Circulation Society http://www.j-circ.or.jp Regulation of Mitochondrial Dynamics and Cell Fate Rimpy Dhingra, PhD; Lorrie A. Kirshenbaum, PhD Though the mitochondrion was initially identified as a key organelle essentially required for energy production and oxidative metabolism, there is considerable evidence that mitochondria are intimately involved in regulating vital cellular processes, such as programmed cell death, proliferation and autophagy. Discovery of mitochondrial “shaping proteins” (Dynamin-related protein (Drp), mitofusins (Mfn) etc.) has revealed that mitochondria are highly dynamic organelles continually changing morphology by fission and fusion processes. Several human pathologies, including cancer, Parkinson’s disease, Alzheimer’s disease and cardiovascular diseases, have been linked to abnormalities in proteins that govern mitochondrial fission or fusion respectively. Notably, in the context of the heart, defects in mitochondrial dynamics resulting in too many fused and/or fragmented mitochondria have been associated with impaired cardiac development, autophagy, and contractile dysfunction. Understanding the mechanisms that govern mitochondrial fission/ fusion is paramount in developing new treatment strategies for human diseases in which defects in fission or fusion is the primary underlying defect. Here, we provide a comprehensive overview of the cellular targets and molecular signal- ing pathways that govern mitochondrial dynamics -

Mitochondrial Dynamic Abnormalities in Alzheimer's Disease Sirui Jiang Case Western Reserve University
MITOCHONDRIAL DYNAMIC ABNORMALITIES IN ALZHEIMER’S DISEASE by SIRUI JIANG Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy Dissertation Advisor: Dr. Xiongwei Zhu Department of Pathology CASE WESTERN RESERVE UNIVERSITY January 2019 CASE WESTERN RESERVE UNIVERSITY SCHOOL OF GRADUATE STUDIES We hereby approve the thesis/dissertation of SIRUI JIANG Candidate for the degree of Doctor of Philosophy* Dr. Shu Chen (Committee Chair) Dr. Xiongwei Zhu Dr. Xinglong Wang Dr. George Dubyak Dr. Charles Hoppel August 15, 2018 *We also certify that written approval has been obtained for any proprietary material contained therein Table of Contents Table of Contents 1 List of Figures 3 Acknowledgements 5 List of Abbreviations 7 Abstract 10 Chapter 1. Introduction 12 Introduction to Alzheimer’s Disease 13 General Information 13 Pathology 14 Pathogenesis 15 Introduction to Mitochondrial Dynamics 20 Mitochondrial Function and Neuronal Health 20 Mitochondrial Dynamics 21 Mitochondrial Dynamics and Mitochondrial Function 23 Mitochondrial Dynamics and Mitochondrial Transport 24 Mitochondrial Deficits in AD 26 Mitochondrial Dysfunction in AD 26 Aβ and Mitochondrial Dysfunction 27 Mitochondrial Dynamic Abnormalities in AD: Recent Advances 28 Conclusion 34 1 Chapter 2. Mfn2 ablation causes an oxidative stress response and eventual neuronal death in the hippocampus and cortex 36 Abstract 37 Background 39 Methods 43 Results 47 Discussion 54 Figures 60 Chapter 3. DLP1 Cleavage by Calpain in Alzheimer’s Disease 71 Abstract 72 Background 73 Methods 77 Results 80 Discussion 85 Figures 89 Chapter 4. Summary, Discussion and Future Directions 96 References 108 2 List of Figures Figure 2.1 Cre-mediated ablation of Mfn2 expression in the hippocampus and cortex of Mfn2 cKO mice 60 Figure 2.2 Quantification of DLP1 and OPA1 in cKO mice 61 Figure 2.3 Mfn2 ablation caused mitochondrial fragmentation and ultrastructural damage in the hippocampus in vivo as evidenced by electron microscopic analysis. -

CD34+ Acute Myeloid Leukemia Cells with Low Levels of Reactive Oxygen Species Show Increased Expression of Stemness Genes and Ca
LETTERS TO THE EDITOR relapse is frequent, especially in elderly patients. Disease + CD34 acute myeloid leukemia cells with low levels relapse is likely driven by leukemia stem cells that are not of reactive oxygen species show increased affected by chemotherapy and therefore retain their dis- expression of stemness genes and can be targeted ease-initiating properties.1 Further characterization of this by the BCL2 inhibitor venetoclax leukemia stem cell population is therefore highly rele- vant. Acute myeloid leukemia (AML) is a genetically hetero- In hematopoietic cells, the stage of differentiation and geneous disease characterized by the accumulation of metabolic properties are closely linked.2 Hematopoietic immature myeloid blasts in the bone marrow. While con- stem cells characteristically have low levels of mitochon- ventional chemotherapy usually results in initial reduc- drial oxidative metabolism and consequently low levels tion of leukemic blasts in the majority of patients, disease of reactive oxygen species (ROS), which is relevant for A B C D E F G H P=0.057 Figure 1. CD34+ acute myeloid leukemia cells with low levels of reactive oxygen species express stemness-associated genes. (A) Gating strategy for defining isolated acute myeloid leukemia (AML) cells with low or high levels of reactive oxygen species (ROS-low and ROS-high, respectively). CD34+ isolated AML cells were stained with the fluorescence dye CellROX Deep Red and gated for cells with the 15% lowest or highest signal intensity. (B) Representative FACS plots (ROS- low: blue, ROS-high: red) and May-Grünwald Giemsa staining indicating the size of ROS-low and ROS-high CD34+ AML cells (n=4). -

Genome-Wide Synthetic Lethal CRISPR Screen Identifies FIS1 As a Genetic Interactor of ALS-Linked C9ORF72
bioRxiv preprint doi: https://doi.org/10.1101/778118; this version posted September 21, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. Genome-wide synthetic lethal CRISPR screen identifies FIS1 as a genetic interactor of ALS-linked C9ORF72 Noori Chai1,2, Michael S. Haney1, Julien Couthouis1, David W. Morgens1, Alyssa Benjamin1, Kathryn Wu1,2, James Ousey1, Shirleen Fang1, Sarah Finer1, Michael C. Bassik1, Aaron D. Gitler1,* Affiliations: 1. Department of Genetics, Stanford University School of Medicine, Stanford, CA USA 2. Stanford Neurosciences Graduate Program, Stanford University School of Medicine, Stanford, CA, USA *Correspondence to: [email protected] bioRxiv preprint doi: https://doi.org/10.1101/778118; this version posted September 21, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. Abstract Mutations in the C9ORF72 gene are the most common cause of amyotrophic lateral sclerosis (ALS). Both toxic gain of function and loss of function pathogenic mechanisms have been proposed. Accruing evidence from mouse knockout studies point to a role for C9ORF72 as a regulator of immune function. To provide further insight into its cellular function, we performed a genome-wide synthetic lethal CRISPR screen in human myeloid cells lacking C9ORF72. We discovered a strong synthetic lethal genetic interaction between C9ORF72 and FIS1, which encodes a mitochondrial membrane protein involved in mitochondrial fission and mitophagy. -

The Impact of Exercise on Mitochondrial Dynamics and the Role of Drp1 in Exercise Performance and Training Adaptations in Skeletal Muscle
Original Article The impact of exercise on mitochondrial dynamics and the role of Drp1 in exercise performance and training adaptations in skeletal muscle Timothy M. Moore 1,2, Zhenqi Zhou 2, Whitaker Cohn 3, Frode Norheim 4, Amanda J. Lin 2, Nareg Kalajian 2, Alexander R. Strumwasser 2, Kevin Cory 2, Kate Whitney 2, Theodore Ho 2, Timothy Ho 2, Joseph L. Lee 2, Daniel H. Rucker 2, Orian Shirihai 2, Alexander M. van der Bliek 5, Julian P. Whitelegge 3, Marcus M. Seldin 4, Aldons J. Lusis 4,6, Sindre Lee 7, Christian A. Drevon 7,8, Sushil K. Mahata 9,10, Lorraine P. Turcotte 1, Andrea L. Hevener 2,11,* ABSTRACT Objective: Mitochondria are organelles primarily responsible for energy production, and recent evidence indicates that alterations in size, shape, location, and quantity occur in response to fluctuations in energy supply and demand. We tested the impact of acute and chronic exercise on mitochondrial dynamics signaling and determined the impact of the mitochondrial fission regulator Dynamin related protein (Drp)1 on exercise performance and muscle adaptations to training. þ À Methods: Wildtype and muscle-specific Drp1 heterozygote (mDrp1 / ) mice, as well as dysglycemic (DG) and healthy normoglycemic men (control) performed acute and chronic exercise. The Hybrid Mouse Diversity Panel, including 100 murine strains of recombinant inbred mice, was used to identify muscle Dnm1L (encodes Drp1)-gene relationships. Results: Endurance exercise impacted all aspects of the mitochondrial life cycle, i.e. fission-fusion, biogenesis, and mitophagy. Dnm1L gene expression and Drp1Ser616 phosphorylation were markedly increased by acute exercise and declined to baseline during post-exercise recovery. -

Mitochondrial Markers 1
Mitochondrial Markers 1 MITOCHONDRIAL MARKERS www.ptglab.com Mitochondrial Markers 2 INTRODUCTION Mitochondria are important cellular organelles that maintain cellular energy balance, contain key regulators of cell death processes, and play a significant role in cellular oxidative stress generation and maintenance of calcium homeostasis. Links to cancer, apoptosis, autophagy, and hypoxia have brought mitochondria to the forefront of scientific studies in recent years. Knowledge of the subcellular location of a protein may reveal the potential role it plays in a variety of cellular processes. Proteintech®* offers approximately all the antibodies needed for mitochondria research. What’s Inside 3–4 Mitochondrial Markers 5 Citric Acid Cycle 6–7 Mitochondrial Respiratory Complexes 8 Mitochondrial Fission Mitochondrial Fusion 9 Mitochondrial Mediated Apoptosis 10 Mitochondrial Autophagy 11 Mitochondrial Translation Mitochondrial Protein Import 12 Contact Us *Proteintech® and the Proteintech logo are trademarks of Proteintech Group registered in the US Patent and Trademark Office. Mitochondrial Markers 3 Mitochondrial Mitochondria are composed of the inner and outer membranes, the intermembrane space, the cristae, and the matrix, and they contain Markers their own DNA separated from the nucleus. Knowledge of the subcellular location of a protein may reveal the potential role it plays in a variety of cellular processes. Colocalization with one of the organelle-specific markers listed here can confirm the subcellular location of a mitochondrial -

FIS1 Encodes a GA2-Oxidase That Regulates Fruit Firmness in Tomato
ARTICLE https://doi.org/10.1038/s41467-020-19705-w OPEN FIS1 encodes a GA2-oxidase that regulates fruit firmness in tomato Ren Li1,2,7, Shuai Sun1,2,7, Haijing Wang1,2, Ketao Wang3, Hong Yu 4, Zhen Zhou1,2, Peiyong Xin4, Jinfang Chu4, ✉ Tongmin Zhao5, Huanzhong Wang 6, Jiayang Li 4 & Xia Cui 1,2 Fruit firmness is a target trait in tomato breeding because it facilitates transportation and storage. However, it is also a complex trait and uncovering the molecular genetic mechan- 1234567890():,; isms controlling fruit firmness has proven challenging. Here, we report the map-based cloning and functional characterization of qFIRM SKIN 1 (qFIS1), a major quantitative trait locus that partially determines the difference in compression resistance between cultivated and wild tomato accessions. FIS1 encodes a GA2-oxidase, and its mutation leads to increased bioactive gibberellin content, enhanced cutin and wax biosynthesis, and increased fruit firmness and shelf life. Importantly, FIS1 has no unfavorable effect on fruit weight or taste, making it an ideal target for breeders. Our study demonstrates that FIS1 mediates gibberellin catabolism and regulates fruit firmness, and it offers a potential strategy for tomato breeders to produce firmer fruit. 1 Key Laboratory of Biology and Genetic Improvement of Horticultural Crops of the Ministry of Agriculture, Institute of Vegetables and Flowers, Chinese Academy of Agricultural Sciences, Beijing 100081, China. 2 Sino-Dutch Joint Laboratory of Horticultural Genomics, Institute of Vegetables and Flowers, Chinese Academy of Agricultural Sciences, Beijing 100081, China. 3 State Key Laboratory of Subtropical Forest Cultivation, Zhejiang Agriculture and Forestry University, Hangzhou 311300, China. -

Rapamycin Ameliorates Defects in Mitochondrial Fission and Mitophagy in Glioblastoma Cells
International Journal of Molecular Sciences Article Rapamycin Ameliorates Defects in Mitochondrial Fission and Mitophagy in Glioblastoma Cells Paola Lenzi 1,† , Rosangela Ferese 2,† , Francesca Biagioni 2 , Federica Fulceri 3 , Carla L. Busceti 2, Alessandra Falleni 3 , Stefano Gambardella 2,4, Alessandro Frati 2,5 and Francesco Fornai 1,2,* 1 Department of Translational Research and New Technologies in Medicine and Surgery, University of Pisa, Via Roma 55, 56126 Pisa, Italy; [email protected] 2 I.R.C.C.S. Neuromed, Via Atinense 18, 86077 Pozzilli (IS), Italy; [email protected] (R.F.); [email protected] (F.B.); [email protected] (C.L.B.); [email protected] (S.G.); [email protected] (A.F.) 3 Department of Clinical and Experimental Medicine University of Pisa, via Roma 55, 56126 Pisa, Italy; [email protected] (F.F.); [email protected] (A.F.) 4 Department of Biomolecular Sciences, University of Urbino “Carlo Bo”, Piazza S. Andrea 34, 61029 Urbino (PU), Italy 5 Neurosurgery Division, Human Neurosciences Department, Sapienza University, 00135 Roma, Italy. * Correspondence: [email protected] or [email protected] † These authors equally contributed to the present work. Abstract: Glioblastoma (GBM) cells feature mitochondrial alterations, which are documented and quantified in the present study, by using ultrastructural morphometry. Mitochondrial impairment, which roughly occurs in half of the organelles, is shown to be related to mTOR overexpression and autophagy suppression. The novelty of the present study consists of detailing an mTOR-dependent mitophagy occlusion, along with suppression of mitochondrial fission. These phenomena contribute Citation: Lenzi, P.; Ferese, R.; to explain the increase in altered mitochondria reported here. -

The Role of Parkin and Mitophagy in Acetaminophen and Alcohol-Induced Liver Injuries
The Role of Parkin and Mitophagy in Acetaminophen and Alcohol-induced Liver Injuries By Jessica A. Williams Submitted to the graduate degree program in Pharmacology, Toxicology, and Therapeutics and the Graduate Faculty of the University of Kansas in partial fulfillment of the requirements for the degree of Doctor of Philosophy. ________________________________ Chairperson Dr. Wen-Xing Ding, PhD ________________________________ Dr. Hartmut Jaeschke, PhD ________________________________ Dr. Michele Pritchard, PhD ________________________________ Dr. Udayan Apte, PhD ________________________________ Dr. Benyi Li, MD, PhD Date Defended: May 28th, 2015 The Dissertation Committee for Jessica A. Williams certifies that this is the approved version of the following dissertation: The Role of Parkin and Mitophagy in Acetaminophen and Alcohol-induced Liver Injuries ________________________________ Chairperson Dr. Wen-Xing Ding Date approved: June 8th, 2015 ii Abstract Acetaminophen (APAP) is the leading cause of acute liver failure in the United States, and alcoholic liver disease (ALD) is a worldwide health problem that claims two million lives per year. Currently, the only cure for either disease is liver transplantation in severe disease states. Therefore, new therapeutic options for treatment of these liver diseases are greatly needed. To develop new therapeutic options, the mechanisms involved in APAP and alcohol-induced liver toxicities must be better understood. We previously demonstrated that autophagy was protective against both APAP and alcohol-induced liver injuries by removing damaged mitochondria by mitophagy, which is a selective form of autophagy specific for mitochondria. However, the mechanisms for induction of mitophagy in the liver are unknown. Parkin is an E3 ubiquitin ligase that is well known to be required for mitophagy induction in mammalian cell models after mitochondrial depolarization.