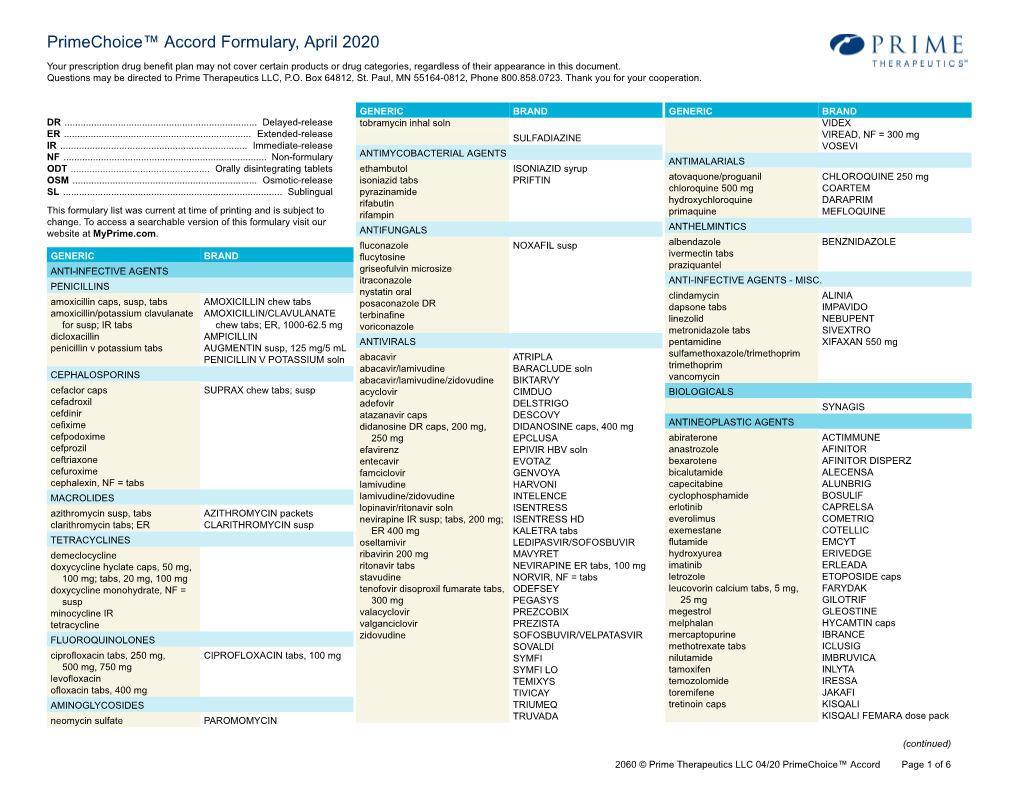
Primechoice™ Accord Formulary, April 2020
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rectal Suppository & Enema Administration to Administration Unlicensed Assistive Personnel (UAP) Module/Skill Checklist
Delegation of Medication Rectal Suppository & Enema Administration to Administration Unlicensed Assistive Personnel (UAP) Module/Skill Checklist Objective At the completion of this module, the UAP should be able to administer rectal suppository & enema medications. NOTE: 1) The RN or LPN is permitted to delegate ONLY after application of all components of the NCBON Decision Tree for Delegation to UAP and after careful consideration that delegation is appropriate: a) for this client, b) with this acuity level, c) with this individual UAP’s knowledge and experience, and d) now (or in the time period being planned). 2) Successful completion of the “Infection Control” module by the UAP should be documented prior to instruction in medication administration by this or ANY route. Procedure for: SUPPOSITORY 1. Perform skills in General Medication Administration Checklist. 2. Provide privacy. Have client void before procedure. 3. Put on clean gloves. 4. Position the client on their left (preferred) side with the top leg bent slightly. 5. Remove the foil or wrapper from the suppository, if present. 6. Apply a small amount of lubricant to the suppository and your gloved index finger on your dominant hand – the hand holding the suppository. 7. Separate the buttocks with your gloved, non dominant hand. 8. Ask the client to breathe slowly and deeply through the mouth. 9. Place tip of suppository against anus and gently insert the suppository about 4- inches along the rectal wall. Avoid putting the suppository in stool. 10. After removing your finger, squeeze buttocks together for a few minutes to help client hold in the suppository for as long as possible. -

California Essential Drug List
California Essential Drug List The Essential Drug List (formulary) includes a list of drugs covered by Health Net. The drug list is updated at least monthly and is subject to change. All previous versions are no longer in effect. You can view the most current drug list by going to our website at www.healthnet.com. Refer to Evidence of Coverage or Certificate of Insurance for specific cost share information. For California Individual & Family Plans: Drug Lists Select Health Net Large Group – Formulary (pdf). For Small Business Group: Drug Lists Select Health Net Small Business Group – Formulary (pdf). NOTE: To search the drug list online, open the (pdf) document. Hold down the “Control” (Ctrl) and “F” keys. When the search box appears, type the name of your drug and press the “Enter” key. If you have questions or need more information call us toll free. California Individual & Family Plans (off-Exchange) If you have questions about your pharmacy coverage call Customer Service at 1-800-839-2172 California Individual & Family Plans (on-Exchange) If you have questions about your pharmacy coverage call Customer Service at 1-888-926-4988 Hours of Operation 8:00am – 7:00pm Monday through Friday 8:00am – 5:00pm Saturday Small Business Group If you have questions about your pharmacy coverage call Customer Service at 1-800-361-3366 Hours of Operation 8:00am – 6:00pm Monday through Friday Updated September 1, 2021 Health Net of California, Inc. and Health Net Life Insurance Company are subsidiaries of Health Net, LLC and Centene Corporation. Health Net is a registered service mark of Health Net, LLC Table of Contents What If I Have Questions Regarding My Pharmacy Benefit? ................................... -

Bowel Preparation Instructions Fleet Enema
66 BOWEL PREPARATION INSTRUCTIONS FLEET ENEMA Please read these instructions before you have your enema and follow carefully. If you need more advice before using the enema, please telephone the Endoscopy Unit on Tel: 01246 512197 You have been given a phosphate enema, which should be kept and used at room temperature. This is to be used on the day you are coming to hospital for your flexible sigmoidoscopy procedure. Instructions: • The enema must be given at least one hour before you leave home to attend hospital. e.g. if your appointment is at 2.00pm and you will be leaving at 1.30pm you must give the enema at 12.30pm. • To give the enema, you need to lie down on your side with both knees bent and pulled up towards your chest. • Remove the enema cap and throw it away. • Relax and gently insert the full length of the nozzle into your rectum (back passage). • Do this without using undue force. The tip of the nozzle is already lubricated to make insertion easier • Squeeze the bottle until it is completely empty. The bottle may be rolled up (like a tube of toothpaste) ensure it empties. Then gently withdraw the bottle while still squeezing to keep it rolled up. • Hold the liquid in your back passage for as long as you can (by clenching your buttocks) 66 MAKE SURE YOU ARE CLOSE TO A TOILET AS THE ENEMA WILL RESULT IN A RAPID EMPTYING OF YOUR BOWELS • Return the used enema to its carton and throw away safely in your domestic waste. -

About Your Surgery Pre-Operative Instructions Prior to Surgery Post-Operative Instructions How to Reach Us
www.fpminstitute.com About Your Surgery Pre-operative Instructions Prior to Surgery Post-operative Instructions How to Reach Us www.fpminstitute.com Pre-operative Instructions Please Note Patient Name: Procedure: Vaginal Colpopexy Enterocele / Rectocele Repair A physician at The Institute for Female Pelvic Medicine & Sacral Colpopexy Colpocleisis / Colpectomy Reconstructive Surgery is available for emergencies 24 hours per day. Hysterectomy Removal / Revision Uterosacral Vault Suspension InterStim® Office hours are Monday through Friday, 8 a.m. to 4 p.m. Colporrhaphy Pubovaginal Sling After hours and on weekends, you can leave non-urgent messages Other: that will be returned the next business day. For urgent situations, Surgery Date: the answering service can page the physician on call. Medical Clearance Blood work at Lab Blood work on Admit. You are required to be medically cleared for surgery by your primary care physician and/or cardiologist within 30 days of your scheduled surgery. You have been given a medical clearance form that your physician will need to complete, as well as a script for an EKG and bloodwork. Please contact your physician and schedule your medical clearance appointment for the week of (not before this www.fpminstitute.com date). If your physician sends you to a lab for bloodwork, we suggest you do this at least two to three days before your appointment, but not before the date indicated above. We will need copies of your test results and completed medical clearance form faxed to us at the number on the form at least three (3) business days prior to your surgery. If you have any questions, please contact our nursing department. -

2017 List of Rule Based Prescription Drugs
2017 List of Rule Based Prescription Drugs Rule Explanation of Rule Prior Authorization Certain medications require a prior authorization. The medications requiring prior authorization are listed below. Your physician will need to complete a prior authorization form to determine if the medication will be approved for your medical condition. Contact EnvisionRXOptions Customer Service Help Desk at 1-800-361-4542 to start the Prior Authorization process. Quantity Limit Quantity limits are clinically recommended limits put in place to help ensure safe utilization of medication. Certain medications are subject to a quantity limit. If you are taking one of the medications mandating a quantity limit and the amount you take does not exceed the limit, you do not need to do anything. If you are in need of a medication that requires a higher quantity than that which is listed, you will need to have your prescribing physician submit a letter of medical necessity explaining why it is medically necessary for you to be on the exact dosage and quantity. You or your prescribing physician can begin the letter of medical necessity process by contacting EnvisionRXOptions Helpdesk at 1-800-361-4542. New-to-Market Medications Any medication approved to enter the market will only be covered after a clinical review decision has been made by the Envision Pharmacy and Therapeutics Committee who reviews safety, efficacy and cost information to determine whether or not the medication will be covered. If you attempt to fill or claim a non-covered New-to-Market medication, the claim will reject due to the medications New- to-Market classification. -

Medi-Cal Formulary May 2020
Formulary Medi-Cal MAY 2020 The IEHP Medi-Cal Formulary is subject to change throughout the year. All previous versions of the formulary are no longer in effect once a new formulary is available. The IEHP Medi-Cal Formulary can be found at: www.iehp.org/en/members/medical Last Updated: 04/17/2020 Inland Empire Health Plan (IEHP) Medi-Cal Formulary Table of Contents A . Foreword ..................................................................................................................... 2 B. IEHP Member Services .............................................................................................. 2 C. How to Use the Formulary .......................................................................................... 2 D. Prescription Coverage ................................................................................................. 3 D1. Quantity Limits .................................................................................................... 3 D2. Step Therapy ........................................................................................................ 3 E. Filling a Prescription and IEHP’s Pharmacy Network ............................................... 4 F. Definitions ................................................................................................................... 4 G. List of Covered Drugs .............................................................................................. 10 H. Index ....................................................................................................................... -

Inhalation Drug Delivery Devices: Technology Update
Medical Devices: Evidence and Research Dovepress open access to scientific and medical research Open Access Full Text Article REVIEW Inhalation drug delivery devices: technology update Mariam Ibrahim Abstract: The pulmonary route of administration has proven to be effective in local and Rahul Verma systemic delivery of miscellaneous drugs and biopharmaceuticals to treat pulmonary and non- Lucila Garcia-Contreras pulmonary diseases. A successful pulmonary administration requires a harmonic interaction between the drug formulation, the inhaler device, and the patient. However, the biggest single Department of Pharmaceutical Sciences, College of Pharmacy, problem that accounts for the lack of desired effect or adverse outcomes is the incorrect use The University of Oklahoma Health of the device due to lack of training in how to use the device or how to coordinate actuation Sciences Center, Oklahoma City, and aerosol inhalation. This review summarizes the structural and mechanical features of OK, USA aerosol delivery devices with respect to mechanisms of aerosol generation, their use with different formulations, and their advantages and limitations. A technological update of the current state-of-the-art designs proposed to overcome current challenges of existing devices is also provided. Keywords: pulmonary delivery, asthma, nebulizers, metered dose inhaler, dry powder inhaler Introduction Inhalation therapy has been used for thousands of years, albeit in a different form and use. Inhalation therapy was practiced by ancient civilizations in Egypt, Greece, India, and People’s Republic of China as evidenced by different artifacts displayed in museums, that may be considered the first used inhalation devices.1,2 Currently, inhalation therapy is the best option for lung diseases like asthma, cystic fibrosis, and chronic obstructive pulmonary disease (COPD). -

Olodaterol Monograph
Olodaterol Monograph Olodaterol (Striverdi Respimat) National Drug Monograph VA Pharmacy Benefits Management Services, Medical Advisory Panel, and VISN Pharmacist Executives The purpose of VA PBM Services drug monographs is to provide a comprehensive drug review for making formulary decisions. Updates will be made when new clinical data warrant additional formulary discussion. Documents will be placed in the Archive section when the information is deemed to be no longer current. FDA Approval Information Description/Mechan Olodaterol is a long-acting beta2-adrenergic agonist (LABA). Binding to and activating ism of Action beta2-adrenoceptors in the airways results in stimulation of intracellular adenyl cyclase, an enzyme that mediates the synthesis of cyclic-3’, 5’ adenosine monophosphate (cAMP). Elevated levels of cAMP induce bronchodilation by relaxation of airway smooth muscle cells. Indication(s) Under Long-term once daily maintenance bronchodilator treatment of airflow obstruction in Review patients with COPD including chronic bronchitis and/or emphysema Dosage Form(s) Inhalation spray for oral inhalation via Respimat (a soft-mist inhaler) Under Review The soft-mist inhalers (SMI) provide multi-dose medication using liquid formulations similar to that used in nebulizers and are propellant-free. Presently, Respimat is the only SMI commercially available for clinical use. The soft mist is released at a slower velocity and has more prolonged spray duration than the mist produced from pressurized metered dose inhalers (pMDIs). Pressurized MDIs require coordination of actuation with inhalation which may be difficult for some patients partly due to the rapid speed at which the drug is delivered and the short duration of the mist. -

Preventive Drug List
navitus.com Share a Clear View Preventive Drug List Updated July 2021 Your health plan is making an effort to reduce your health care costs by giving you tools to help you stay healthy and productive. Below are the medications included on your Preventive Drug List. These medications help protect against or manage some high risk medical conditions. Taking these medications as directed by your prescriber can help avoid serious health problems. That may mean fewer doctor visits and hospitalizations, reducing your total health care costs. In the drug list below, generic drugs are shown in lowercase type. Brand name drugs are shown in uppercase type. Antiasthmatic/Bronchodilators theophylline soln ADVAIR DISKUS INHALER *generic Wixela only for wixela inhaler *brand Advair Diskus only for non-HDHP plans* HDHPs* ADVAIR HFA INHALER Anticoagulant albuterol/ipratropium neb soln ELIQUIS TAB aminophylline tab PRADAXA CAP ARNUITY ELLIPTA INHALER warfarin tab ASMANEX HFA INHALER XARELTO TAB ASMANEX INHALER Antidiabetics budesonide inh susp acarbose tab FLOVENT DISKUS INHALER chlorpropamide tab FLOVENT HFA INHALER glimepiride tab fluticasone/salmeterol inhaler glipizide ER tab ipratropium neb soln glipizide tab METAPROTERENOL SYRUP glipizide/metformin tab montelukast chew tab glyburide micronized tab montelukast tab glyburide tab THEOCHRON glyburide/metformin tab theophylline CR tab metformin ER tab theophylline ER tab metformin tab • Note: The list is subject to change and not all drugs listed may be covered on your formulary. Please refer to your Navitus -

Connecticut Medicaid
ACNE AGENTS, TOPICAL ‡ ANGIOTENSIN MODULATOR COMBINATIONS ANTICONVULSANTS, CONT. CONNECTICUT MEDICAID (STEP THERAPY CATEGORY) AMLODIPINE / BENAZEPRIL (ORAL) LAMOTRIGINE CHEW DISPERS TAB (not ODT) (ORAL) (DX CODE REQUIRED - DIFFERIN, EPIDUO and RETIN-A) AMLODIPINE / OLMESARTAN (ORAL) LAMOTRIGINE TABLET (IR) (not ER) (ORAL) Preferred Drug List (PDL) ACNE MEDICATION LOTION (BENZOYL PEROXIDE) (TOPICAL)AMLODIPINE / VALSARTAN (ORAL) LEVETIRACETAM SOLUTION, IR TABLET (not ER) (ORAL) • The Connecticut Medicaid Preferred Drug List (PDL) is a BENZOYL PEROXIDE CREAM, WASH (not FOAM) (TOPICAL) OXCARBAZEPINE TABLET (ORAL) listing of prescription products selected by the BENZOYL PEROXIDE 5% and 10% GEL (OTC) (TOPICAL) ANTHELMINTICS PHENOBARBITAL ELIXIR, TABLET (ORAL) Pharmaceutical and Therapeutics Committee as efficacious, BENZOYL PEROXIDE 6% CLEANSER (OTC) (TOPICAL) ALBENDAZOLE TABLET (ORAL) PHENYTOIN CHEW TABLET, SUSPENSION (ORAL) safe and cost effective choices when prescribing for HUSKY CLINDAMYCIN PH 1% PLEGET (TOPICAL) BILTRICIDE TABLET (ORAL) PHENYTOIN SOD EXT CAPSULE (ORAL) A, HUSKY C, HUSKY D, Tuberculosis (TB) and Family CLINDAMYCIN PH 1% SOLUTION (not GEL or LOTION) (TOPICAL)IVERMECTIN TABLET (ORAL) PRIMIDONE (ORAL) Planning (FAMPL) clients. CLINDAMYCIN / BENZOYL PEROXIDE 1.2%-5% (DUAC) (TOPICAL) SABRIL 500 MG POWDER PACK (ORAL) • Preferred or Non-preferred status only applies to DIFFERIN 0.1% CREAM (TOPICAL) (not OTC GEL) (DX CODE REQ.) ANTI-ALLERGENS, ORAL SABRIL TABLET (ORAL) those medications that fall within the drug classes DIFFERIN -

Therapeutic Enema for Intussusception
Therapeutic Enema for Intussusception Therapeutic enema is used to help identify and diagnose intussusception, a serious disorder in which one part of the intestine slides into another in a telescoping manner and causes inflammation and an obstruction. Intussusception often occurs at the junction of the small and large intestine and most commonly occurs in children three to 24 months of age. A therapeutic enema using air or a contrast material solution may be performed to create pressure within the intestine and "un-telescope" the intussusception while relieving the obstruction. This exam is usually performed on an emergency basis. Tell your doctor about your child's recent illnesses, medical conditions, medications and allergies, especially to barium or iodinated contrast materials. Your child may be asked to wear a gown and remove any objects that might interfere with the x-ray images. An ultrasound may be performed to help confirm the diagnosis. What is a Therapeutic Enema for Intussusception? What is intussusception? Intussusception is a serious disorder in which one part of the intestine slides into another part of the intestine, similar to a collapsing telescope. The intestine becomes inflamed and swollen and can cause an obstruction or blockage. Symptoms can include severe abdominal pain, fever, vomiting or abnormal stools. Intussusception may occur anywhere along the gastrointestinal tract; however, it often occurs at the junction of the small and large intestine. The condition most commonly occurs in children three months to 24 months of age. Intussusception is a medical/surgical emergency. If your child has some or all of the symptoms of intussusception, you should call your physician or an emergency medical professional immediately. -

Comparative Study of Enema Retention and Preference in Ulcerative Colitis J R Ingram, J Rhodes, B K Evans, R G Newcombe, G a O Thomas
594 Postgrad Med J: first published as 10.1136/pgmj.2004.031690 on 2 September 2005. Downloaded from ORIGINAL ARTICLE Comparative study of enema retention and preference in ulcerative colitis J R Ingram, J Rhodes, B K Evans, R G Newcombe, G A O Thomas ............................................................................................................................... Postgrad Med J 2005;81:594–598. doi: 10.1136/pgmj.2004.031690 Background: Therapeutic enemas are often used to treat active colitis but their retention may be limited because of urgency to defecate. Some preparations may be better retained and tolerated than others See end of article for because of their physical properties. authors’ affiliations ....................... Aim: To compare patient preference and retention of four therapeutic enemas, including a nicotine enema, in patients with ulcerative colitis (UC). Correspondence to: Methods: Twenty four patients with active UC received the four trial enemas—corticosteroid, 5-amino Dr G A O Thomas, Department of salicylate (5-ASA), and nicotine liquid enemas and a corticosteroid foam, in a randomised order, taking Gastroenterology, one enema on each of four successive nights. Patients scored them 1 to 4 for ease of administration and University Hospital of retention, degree of abdominal bloating, and for their overall preference. Wales, Heath Park, Cardiff CF14 4XW, UK; Gareth. Results: Fifteen patients rated nicotine their overall favourite or second favourite, compared with 14 for [email protected]. corticosteroid foam and 11 for 5-ASA and corticosteroid liquids, but this was not significant (p = 0.302). nhs.uk Overall, there was no significant difference in overnight retention. However, the nicotine enema tended to be less well retained in patients with milder urgency but a higher proportion retained it overnight with Submitted 16 December 2004 more severe urgency (p = 0.031 compared with 5-ASA enema).