Thesis Shorebird Habitat Use and Abundance Estimates
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Upland Sandpiper: a Flagship for Jack Pine Barrens Restoration in the Upper Midwest? R
RESEARCH ARTICLE Upland Sandpiper: A Flagship for Jack Pine Barrens Restoration in the Upper Midwest? R. Gregory Corace III, Jacob L. Korte, Lindsey M. Shartell and Daniel M. Kashian ABSTRACT Fire-dependent ecosystems have been altered across much of North America, and their restoration has the potential to affect many wildlife species, including those of regional conservation priority such as the upland sandpiper (UPSA, Bartra- mia longicauda). In the Upper Midwest, fire-dependent jack pine (Pinus banksiana) barrens were once common and are now a focus of restoration by state, federal, and non-government agencies and organizations. Given UPSA’s association with terrestrial ecosystems such as pastures, hayfields, and barrens, we determined the location of UPSA-occupied areas across multiple states, with special focus on Michigan, to illustrate distributional relationships with specific ecoregions, soils, and land covers while considering what role the species may have as a flagship for barrens restoration. With the exception of Michigan, UPSA-occupied areas in all states studied had greater proportions of agricultural land (National Land Cover Data: pasture/hay and cultivated crops) than other openland cover types. In Michigan, 66% of long-term occupied areas were found in the northern Lower Peninsula, and most often consisted of anthropogenic grasslands pro- viding stable habitat on higher-quality soils. In contrast, short-term occupied areas had a greater proportion of native openlands that were often located on poorer, xeric soils associated with jack pine ecosystems that succeed to closed- canopy forests or shrublands in the absence of fire. Openlands with no UPSA breeding evidence were characterized by intensive agriculture (row crops). -

2020 Mississippi Bird EA
ENVIRONMENTAL ASSESSMENT Managing Damage and Threats of Damage Caused by Birds in the State of Mississippi Prepared by United States Department of Agriculture Animal and Plant Health Inspection Service Wildlife Services In Cooperation with: The Tennessee Valley Authority January 2020 i EXECUTIVE SUMMARY Wildlife is an important public resource that can provide economic, recreational, emotional, and esthetic benefits to many people. However, wildlife can cause damage to agricultural resources, natural resources, property, and threaten human safety. When people experience damage caused by wildlife or when wildlife threatens to cause damage, people may seek assistance from other entities. The United States Department of Agriculture, Animal and Plant Health Inspection Service, Wildlife Services (WS) program is the lead federal agency responsible for managing conflicts between people and wildlife. Therefore, people experiencing damage or threats of damage associated with wildlife could seek assistance from WS. In Mississippi, WS has and continues to receive requests for assistance to reduce and prevent damage associated with several bird species. The National Environmental Policy Act (NEPA) requires federal agencies to incorporate environmental planning into federal agency actions and decision-making processes. Therefore, if WS provided assistance by conducting activities to manage damage caused by bird species, those activities would be a federal action requiring compliance with the NEPA. The NEPA requires federal agencies to have available -

The All-Bird Bulletin
Advancing Integrated Bird Conservation in North America Spring 2014 Inside this issue: The All-Bird Bulletin Protecting Habitat for 4 the Buff-breasted Sandpiper in Bolivia The Neotropical Migratory Bird Conservation Conserving the “Jewels 6 Act (NMBCA): Thirteen Years of Hemispheric in the Crown” for Neotropical Migrants Bird Conservation Guy Foulks, Program Coordinator, Division of Bird Habitat Conservation, U.S. Fish and Bird Conservation in 8 Wildlife Service (USFWS) Costa Rica’s Agricultural Matrix In 2000, responding to alarming declines in many Neotropical migratory bird popu- Uruguayan Rice Fields 10 lations due to habitat loss and degradation, Congress passed the Neotropical Migra- as Wintering Habitat for tory Bird Conservation Act (NMBCA). The legislation created a unique funding Neotropical Shorebirds source to foster the cooperative conservation needed to sustain these species through all stages of their life cycles, which occur throughout the Western Hemi- Conserving Antigua’s 12 sphere. Since its first year of appropriations in 2002, the NMBCA has become in- Most Critical Bird strumental to migratory bird conservation Habitat in the Americas. Neotropical Migratory 14 Bird Conservation in the The mission of the North American Bird Heart of South America Conservation Initiative is to ensure that populations and habitats of North Ameri- Aros/Yaqui River Habi- 16 ca's birds are protected, restored, and en- tat Conservation hanced through coordinated efforts at in- ternational, national, regional, and local Strategic Conservation 18 levels, guided by sound science and effec- in the Appalachians of tive management. The NMBCA’s mission Southern Quebec is to achieve just this for over 380 Neo- tropical migratory bird species by provid- ...and more! Cerulean Warbler, a Neotropical migrant, is a ing conservation support within and be- USFWS Bird of Conservation Concern and listed as yond North America—to Latin America Vulnerable on the International Union for Conser- Coordination and editorial vation of Nature (IUCN) Red List. -

Biogeographical Profiles of Shorebird Migration in Midcontinental North America
U.S. Geological Survey Biological Resources Division Technical Report Series Information and Biological Science Reports ISSN 1081-292X Technology Reports ISSN 1081-2911 Papers published in this series record the significant find These reports are intended for the publication of book ings resulting from USGS/BRD-sponsored and cospon length-monographs; synthesis documents; compilations sored research programs. They may include extensive data of conference and workshop papers; important planning or theoretical analyses. These papers are the in-house coun and reference materials such as strategic plans, standard terpart to peer-reviewed journal articles, but with less strin operating procedures, protocols, handbooks, and manu gent restrictions on length, tables, or raw data, for example. als; and data compilations such as tables and bibliogra We encourage authors to publish their fmdings in the most phies. Papers in this series are held to the same peer-review appropriate journal possible. However, the Biological Sci and high quality standards as their journal counterparts. ence Reports represent an outlet in which BRD authors may publish papers that are difficult to publish elsewhere due to the formatting and length restrictions of journals. At the same time, papers in this series are held to the same peer-review and high quality standards as their journal counterparts. To purchase this report, contact the National Technical Information Service, 5285 Port Royal Road, Springfield, VA 22161 (call toll free 1-800-553-684 7), or the Defense Technical Infonnation Center, 8725 Kingman Rd., Suite 0944, Fort Belvoir, VA 22060-6218. Biogeographical files o Shorebird Migration · Midcontinental Biological Science USGS/BRD/BSR--2000-0003 December 1 By Susan K. -

Grassland Birds in Northeastern Illinois
Birdwatching at Midewin The U.S. Department of Agriculture (USDA) prohibits discrimination in all its programs and activities on the basis of race, color, national origin, age, disability, and where applicable, sex, marital status, familial status, parental status, religion, sexual orientation, genetic information, political beliefs, reprisal, or because all or part of an individual’s income is derived from any public assistance program. (Not all prohibited bases apply to all programs.) Persons with disabilities who require alternative means for communication of program information (Braille, large print, audiotape, etc.) should contact USDA's TARGET Center at (202) 720-2600 (voice and TDD). To file a complaint of discrimination, write USDA, Director, Office of Civil Rights 1400 Independence Avenue SW, Washington, D.C. 20250-9410 or call (800) 795-3272 (voice) or (202) 720-6382 (TDD). USDA is an equal opportunity provider and employer. Midewin National Tallgrass Prairie 30239 S. State Route 53 Wilmington, IL 60481 (815) 423-6370 www.fs.fed.us/mntp/ Midewin National Tallgrass Prairie Brochure design by Gammon Group Bird Species and Habitats at the Midewin National Tallgrass Prairie Midewin, only 40 miles southwest of Chicago, represents the largest contiguous holding of public lands in the greater Chicago region. Bird watching Watchingor birding is a $25 billion industry that As most of the property consists of large grassland fields, Midewin was, according to a survey conducted by the United supports what is arguably the largest and most diverse community States Fish and Wildlife Service, enjoyed by over of grassland birds in northeastern Illinois. Analyses of long-term 50 million Americans in the year 2001. -
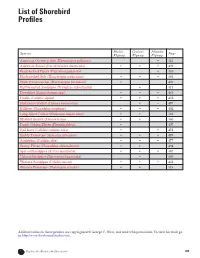
List of Shorebird Profiles
List of Shorebird Profiles Pacific Central Atlantic Species Page Flyway Flyway Flyway American Oystercatcher (Haematopus palliatus) •513 American Avocet (Recurvirostra americana) •••499 Black-bellied Plover (Pluvialis squatarola) •488 Black-necked Stilt (Himantopus mexicanus) •••501 Black Oystercatcher (Haematopus bachmani)•490 Buff-breasted Sandpiper (Tryngites subruficollis) •511 Dowitcher (Limnodromus spp.)•••485 Dunlin (Calidris alpina)•••483 Hudsonian Godwit (Limosa haemestica)••475 Killdeer (Charadrius vociferus)•••492 Long-billed Curlew (Numenius americanus) ••503 Marbled Godwit (Limosa fedoa)••505 Pacific Golden-Plover (Pluvialis fulva) •497 Red Knot (Calidris canutus rufa)••473 Ruddy Turnstone (Arenaria interpres)•••479 Sanderling (Calidris alba)•••477 Snowy Plover (Charadrius alexandrinus)••494 Spotted Sandpiper (Actitis macularia)•••507 Upland Sandpiper (Bartramia longicauda)•509 Western Sandpiper (Calidris mauri) •••481 Wilson’s Phalarope (Phalaropus tricolor) ••515 All illustrations in these profiles are copyrighted © George C. West, and used with permission. To view his work go to http://www.birchwoodstudio.com. S H O R E B I R D S M 472 I Explore the World with Shorebirds! S A T R ER G S RO CHOOLS P Red Knot (Calidris canutus) Description The Red Knot is a chunky, medium sized shorebird that measures about 10 inches from bill to tail. When in its breeding plumage, the edges of its head and the underside of its neck and belly are orangish. The bird’s upper body is streaked a dark brown. It has a brownish gray tail and yellow green legs and feet. In the winter, the Red Knot carries a plain, grayish plumage that has very few distinctive features. Call Its call is a low, two-note whistle that sometimes includes a churring “knot” sound that is what inspired its name. -

Upland Sandpiper (Bartramia Longicauda)
April 2014 Protocol for Incidental Take Permit and Authorization Upland Sandpiper (Bartramia longicauda) Note If carrying out a given protocol is not feasible, or multiple listed species in a given management area pose conflicts, contact the Bureau of Natural Heritage Conservation (NHC) at [email protected]. Staff in NHC will work with species experts and managers to establish an acceptable protocol for a given site that will allow for incidental take without further legal consultation or public notice I. Species Background Information A. Status State Status: Threatened, Species of Greatest Conservation Need USFWS Region 3 Species of Management Concern? Yes Breeding Distribution and Abundance in Wisconsin: Uncommon. Distribution is irregular and localized across the state, tied to areas of sufficiently open grass-dominated landscapes; in such areas it can be locally common. Areas of population concentration occur in the southwest, central, eastern, and northwest parts of the state. Global Range: The contiguous breeding range is east of the Rocky Mountains, bounded on the north from southern Alberta and Saskatchewan, southwest Manitoba, northern Minnesota, Wisconsin and Michigan, southeast Ontario and southern Quebec, south to the central U.S. Great Plains (Kansas, n. Oklahoma, and western Missouri), south-central Illinois and Indiana, southwest Ohio, southern Pennsylvania and northern Virginia. It is locally distributed along the Atlantic coast from Delaware north to the Canadian Maritime Provinces. Isolated breeding populations occur most notably in Alaska, the Yukon and Northwest Territories, as well as in Oregon and Washington states. The core wintering range of the Upland Sandpiper is in South America, east of Andes Mountains. -

Migratory Shorebird Guild
Migratory Shorebird Guild Piping Plover Charadrius melodus Sanderling Calidris alba Semipalmated Plover Charadrius semipalmatus Red Knot Calidris canutus Black-bellied Plover Pluvialis squatarola Marbled Godwit Limosa fedoa American Golden Plover Pluvialis dominica Buff-breasted Sandpiper Tryngites subruficollis Wimbrel Numenius phaeopus White-rumped Sandpiper Calidris fuscicollis Long-billed Curlew Numenius americanus Pectoral Sandpiper Calidris melanotos Greater Yellowlegs Tringa melanoleuca Purple Sandpiper Calidris maritima Lesser Yellowlegs Tringa flavipes Stilt Sandpiper Calidris himantopus Solitary Sandpiper Tringa solitaria Wilson’s Snipe Gallinago gallinago delicata Spotted Sandpiper Actitis macularia American Avocet Recurvirostra Americana Upland Sandpiper Bartramia longicauda Least Sandpiper Calidris minutilla Semipalmated Sandpiper Calidris pusilla Short-billed Dowitcher Limnodromus griseus Western Sandpiper Calidris mauri Long-billed Dowitcher Limnodromus scolopaceus Dunlin Calidris alpina Contributors: Felicia Sanders and Thomas M. Murphy DESCRIPTION Photograph by SC DNR Taxonomy and Basic Description The migratory shorebird guild is composed of birds in the Charadrii suborder. Migrants in South Carolina represent three families: Scolopacidae (sandpipers), Charadriidae (plovers) and Recurvirostridae (avocets). Sandpipers are the most diverse family of shorebirds. Their tactile foraging strategy encompasses probing in soft mud or sand for invertebrates. Plovers are medium size birds, with relatively short, thick bills and employ a distinctive foraging strategy. They stand, looking for prey and then run to feed on detected invertebrates. Avocets are large shorebirds with long recurved bills and partial webbing between the toes. They feed employing both tactile and visual methods. Shorebirds are characterized by long legs for wading and wings designed for quick flight and transcontinental migrations. Migrations can span continents; for example, red knots migrate from the Canadian arctic to the southern tip of South America. -

54971 GPNC Shorebirds
A P ocket Guide to Great Plains Shorebirds Third Edition I I I By Suzanne Fellows & Bob Gress Funded by Westar Energy Green Team, The Nature Conservancy, and the Chickadee Checkoff Published by the Friends of the Great Plains Nature Center Table of Contents • Introduction • 2 • Identification Tips • 4 Plovers I Black-bellied Plover • 6 I American Golden-Plover • 8 I Snowy Plover • 10 I Semipalmated Plover • 12 I Piping Plover • 14 ©Bob Gress I Killdeer • 16 I Mountain Plover • 18 Stilts & Avocets I Black-necked Stilt • 19 I American Avocet • 20 Hudsonian Godwit Sandpipers I Spotted Sandpiper • 22 ©Bob Gress I Solitary Sandpiper • 24 I Greater Yellowlegs • 26 I Willet • 28 I Lesser Yellowlegs • 30 I Upland Sandpiper • 32 Black-necked Stilt I Whimbrel • 33 Cover Photo: I Long-billed Curlew • 34 Wilson‘s Phalarope I Hudsonian Godwit • 36 ©Bob Gress I Marbled Godwit • 38 I Ruddy Turnstone • 40 I Red Knot • 42 I Sanderling • 44 I Semipalmated Sandpiper • 46 I Western Sandpiper • 47 I Least Sandpiper • 48 I White-rumped Sandpiper • 49 I Baird’s Sandpiper • 50 ©Bob Gress I Pectoral Sandpiper • 51 I Dunlin • 52 I Stilt Sandpiper • 54 I Buff-breasted Sandpiper • 56 I Short-billed Dowitcher • 57 Western Sandpiper I Long-billed Dowitcher • 58 I Wilson’s Snipe • 60 I American Woodcock • 61 I Wilson’s Phalarope • 62 I Red-necked Phalarope • 64 I Red Phalarope • 65 • Rare Great Plains Shorebirds • 66 • Acknowledgements • 67 • Pocket Guides • 68 Supercilium Median crown Stripe eye Ring Nape Lores upper Mandible Postocular Stripe ear coverts Hind Neck Lower Mandible ©Dan Kilby 1 Introduction Shorebirds, such as plovers and sandpipers, are a captivating group of birds primarily adapted to live in open areas such as shorelines, wetlands and grasslands. -

Shorebird Habitat Conservation Strategy
Upper Mississippi River and Great Lakes Region Joint Venture Shorebird Habitat Conservation Strategy May 2007 1 Shorebird Strategy Committee and Members of the Joint Venture Science Team Bob Gates, Ohio State University, Chair Dave Ewert, The Nature Conservancy Diane Granfors, U.S. Fish and Wildlife Service Bob Russell, U.S. Fish and Wildlife Service Bradly Potter, U.S. Fish and Wildlife Service Mark Shieldcastle, Ohio Department of Natural Resources Greg Soulliere, U.S. Fish and Wildlife Service Cover: Long-billed Dowitcher. Photo by Gary Kramer. i Table of Contents Plan Summary................................................................................................................... 1 Acknowledgements...................................................................................................... 2 Background and Context ................................................................................................. 3 Population Status and Trends ......................................................................................... 6 Habitat Characteristics .................................................................................................. 11 Biological Foundation..................................................................................................... 14 Planning Framework.................................................................................................. 14 Migration and Distribution........................................................................................ 15 Limiting -

Birds of Ohio Shores
Birds of Ohio Shores: Diversity, Ecology and Management of Shorebirds in Ohio Woodlands Stewards Friday Morning Webinar, October 2, 2020 From Plovers to Pipers (who dey): A diversity tour of Ohio Shorebirds 2 Large Plovers: 3 Common Plovers: 4 Uncommon Plovers: 5 Avocet and Black-Necked Stilt 6 Greater and Lesser Yellowlegs 7 Solitary and Spotted Sandpipers 8 Willet and Upland Sandpiper 9 Whimbrel 10 Hudsonian and Marbled Godwits 11 Ruddy Turnstone and Sanderling 12 “Peeps” (= Calidris spp.) Hard to identify; they all look alike and often occur in large flocks. 13 Dublin and Pectoral Sandpiper 14 White-rumped and Baird’s Sandpipers 15 Semi-palmated and Least Sandpipers 16 Stilt and Buff-breasted Sandpipers 17 End of the “peeps” 18 Long and Short-billed Dowitcher 19 American Woodcock and Wilson’s Snipe 20 Wilson’s and Red-necked Phalaropes 21 And if that were not enough! 22 Breeding, juvenile, fall and spring plumages! 23 Prebalternate and prebasic molts (all spp. of shorebirds, not limited to peeps) Feathers wear so plumage changes spring to fall. 24 Shorebird Guilds Body Size Leg Length Bill size and shape Foraging behavior Habitat type (wetland zone) 25 Shorebird Guilds Small gleaners (beach, dry mudflat) Small probers (moist mudflat) Large probers (moist mudflat, shallow water) Large gleaners (shallow water) 26 Shorebird Habitats Meadow/Marsh Deep (er) Water Shallow Water Wet Mudflat Dry Mudflat 27 28 Shorebird Habitats Dry mudflat species: Killdeer Baird’s Sandpiper Buff-breasted Sandpiper Black-bellied Plover Golden Plover 29 -

Upland Sandpiper & Endangered Species Bartramia Longicauda
Natural Heritage Upland Sandpiper & Endangered Species Bartramia longicauda Program State Status: Endangered www.mass.gov/nhesp Federal Status: None Massachusetts Division of Fisheries & Wildlife DESCRIPTION: The Upland Sandpiper is a slender, moderate-size shore-bird with a small head, large, shoe- button eye, short and thick dark-brown bill, long, thin neck and relatively long tail. Legs are yellowish. It stands about 12 in (30 cm) tall and has a wingspan of 25 to 27 in (64 to 68 cm). The crown is dark brown with a pale buff crown stripe. The rump, upper tail and wings are much darker than the rest of the bird. Calls include a rapid “quip-ip-ip-ip” alarm call, and a long, drawn-out courtship call which has been described as a windy, whistly, “whiiip-whee-ee-oo.” The sexes are similar. This species often poses with its wings up raised when alighting on utility poles or fence posts. Robbins, C.S., B. Braun, and H.S. Zim. Birds of North America. Golden Press, HABITAT IN MASSACHUSETTS: The Upland New York. 1966. Sandpiper inhabits large expanses of open grassy uplands, wet meadows, old fields, and pastures. In ECOLOGY AND BEHAVIOR: The Uplands Massachusetts it is restricted to open expanses of grassy Sandpiper returns to its breeding habitat in fields, hay fields, and mown grassy strips adjacent to Massachusetts mid-April to early May. The birds arrive runways and taxiways of airports and military bases. already paired and usually return to the same area year They need feeding and loafing areas as well as nesting after year.